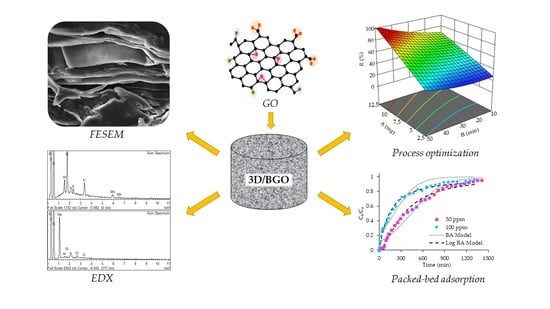
Remediation of Amitriptyline Pharmaceutical Wastewater by Heteroatom-Doped Graphene Oxide: Process Optimization and Packed-Bed Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of 3D/BGO Adsorbent
2.4. Characterization Tests
2.5. HPLC Analysis
2.6. CCD Experimental Design and Modeling
2.7. Fixed-Bed Adsorption Tests
2.8. Column Adsorption Modeling
2.9. Regeneration Test
3. Results and Discussion
3.1. Characterization of GO
3.1.1. FESEM Analysis
3.1.2. EDX Analysis
3.1.3. TGA
3.1.4. FTIR Analysis
| Wavenumber Range (cm−1) | Bond | Wavenumber (cm−1) | Reference | |
|---|---|---|---|---|
| Before Adsorption | After Adsorption | |||
| 3200–3550 | O–H stretching | 3218 | 3205 | [39,41,42] |
| 2840–3000 | C–H stretching | 2921 | 2925 | |
| 1566–1650 | C=C stretching | 1583 | 1585 | |
| 395–1440 | O–H bonding | 1414 | - | |
| 1310–1390 | O–H bending | 1324 | - | |
| 1300–1000 | C–O stretching | 1056 | 1062 | |
| 1022 | 1013 | |||
| 930–1284 | C–N, C–B, B–O stretching | 930 | - | |
3.1.5. XPS Analysis
3.2. CCD Model Generation
3.2.1. Model Verification
3.2.2. Response Surface Analysis and Process Optimization
3.3. Fixed-Bed Column Studies
3.3.1. Effect of Column Parameters
3.3.2. Breakthrough Curve Modelling
3.4. Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umaharan, T.; Sivayokan, S.; Sivansuthan, S. Amitriptyline Dependence and Its Associations: A Case Report and Literature Review. Case Rep. Psychiatry 2021, 2021, 6647952. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, C.; Wu, X.; Han, Z.; Zhang, S.; Chen, K.; Qiu, X. Exposure to amitriptyline induces persistent gut damages and dysbiosis of the gut microbiota in zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 260, 109417. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shah, J.C.; Hwang, S.S. Pharmacokinetic and pharmacodynamic characterization of OROS and immediate-release amitriptyline. Br. J. Clin. Pharmacol. 1999, 48, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, C.; Han, Z.; Chen, K.; Wu, X.; Qiu, X. Combined exposure to microplastics and amitriptyline caused intestinal damage, oxidative stress and gut microbiota dysbiosis in zebrafish (Danio rerio). Aquat. Toxicol. 2023, 260, 106589. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Bhogal, S.; Grover, A.; Malik, A.K.; Aulakh, J.S. Simultaneous determination of amitriptyline, nortriptyline, and clomipramine in aqueous samples using selective multi-template molecularly imprinted polymers. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100527. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Multi-residue analysis of pharmaceutical compounds in aqueous samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar] [CrossRef]
- Nordin, A.H.; Norfarhana, A.S.; Noor, S.F.M.; Paiman, S.H.; Nordin, M.L.; Husna, S.M.N.; Ilyas, R.A.; Ngadi, N.; Bakar, A.A.; Ahmad, Z.; et al. Recent Advances in Using Adsorbent Derived from Agricultural Waste for Antibiotics and Non-Steroidal Anti-Inflammatory Wastewater Treatment: A Review. Separations 2023, 10, 300. [Google Scholar] [CrossRef]
- Köpping, I.; McArdell, C.S.; Borowska, E.; Böhler, M.A.; Udert, K.M. Removal of pharmaceuticals from nitrified urine by adsorption on granular activated carbon. Water Res. X 2020, 9, 100057. [Google Scholar] [CrossRef]
- Tee, W.T.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Biochar as a remediation solution for pharmaceutical-contaminated wastewater. In BioChar: Applications for Bioremediation of Contaminated Systems; De Gruyter: Berlin, Germany, 2022; p. 373. [Google Scholar]
- Sarti, E.; Chenet, T.; Stevanin, C.; Costa, V.; Cavazzini, A.; Catani, M.; Martucci, A.; Precisvalle, N.; Beltrami, G.; Pasti, L. High-Silica Zeolites as Sorbent Media for Adsorption and Pre-Concentration of Pharmaceuticals in Aqueous Solutions. Molecules 2020, 25, 3331. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Chemmangattuvalappil, N.; Lee, L.Y. Adsorptive removal of salicylic acid from aqueous solutions using new graphene-based nanosorbents. Chem. Eng. Trans. 2015, 45, 1387–1392. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Tee, W.T.; Loh, N.Y.L.; Lai, K.C.; Hanson, S.; Gan, S.; Thangalazhy-Gopakumar, S.; Lee, L.Y. Synthesis of a highly recoverable 3D MnO2/rGO hybrid aerogel for efficient adsorptive separation of pharmaceutical residue. J. Environ. Sci. 2022, 118, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Wang, S.; Tian, C.; Lin, Z. Adsorption of low-concentration mercury in water by 3D cyclodextrin/graphene composites: Synergistic effect and enhancement mechanism. Environ. Pollut. 2019, 252, 1133–1141. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; Achaby, M.E. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Tee, W.T.; Loh, N.Y.L.; Hiew, B.Y.Z.; Chiu, W.S.; Khiew, P.S.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Design and development of a high-performance 3D graphene system for adsorptive removal of organic toxins from wastewater: Mechanisms and process optimization. Chem. Eng. Res. Des. 2023, 195, 132–150. [Google Scholar] [CrossRef]
- Tee, W.T.; Loh, N.Y.L.; Lai, K.C.; Hiew, B.Y.Z.; Gan, S.; Lee, L.Y. Application of 3D heteroatom-doped graphene in adsorptive removal of water pollutants: Review on hydrothermal synthesis and its influencing factors. Sep. Purif. Technol. 2023, 320, 124072. [Google Scholar] [CrossRef]
- El Hadki, A.; Ulucan-Altuntas, K.; El Hadki, H.; Ustundag, C.B.; Kabbaj, O.K.; Dahchour, A.; Komiha, N.; Zrineh, A.; Debik, E. Removal of oxytetracycline by graphene oxide and Boron-doped reduced graphene oxide: A combined density function Theory, molecular dynamics simulation and experimental study. FlatChem 2021, 27, 100238. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Solgi, M.; Steiger, B.G.K.; Wilson, L.D. A Fixed-Bed Column with an Agro-Waste Biomass Composite for Controlled Separation of Sulfate from Aqueous Media. Separations 2023, 10, 262. [Google Scholar] [CrossRef]
- Yu, F.; Pan, J.; Li, Y.; Yang, Y.; Zhang, Z.; Nie, J.; Ma, J. Batch and continuous fixed-bed column adsorption of tetracycline by biochar/MOFs derivative covered with κ-carrageenan/calcium alginate hydrogels. J. Environ. Chem. Eng. 2022, 10, 107996. [Google Scholar] [CrossRef]
- Hethnawi, A.; Alnajjar, M.; Manasrah, A.D.; Hassan, A.; Vitale, G.; Jeong, R.; Nassar, N.N. Metformin Removal from Water Using Fixed-bed Column of Silica-Alumina Composite. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124814. [Google Scholar] [CrossRef]
- Américo-Pinheiro, J.H.P.; Salomão, G.R.; Moreno Paschoa, C.V.; Cruz, I.A.; Isique, W.D.; Romanholo Ferreira, L.F.; Torres, N.H.; Bilal, M.; Iqbal, H.M.N.; Sillanpää, M.; et al. Effective adsorption of diclofenac and naproxen from water using fixed-bed column loaded with composite of heavy sugarcane ash and polyethylene terephthalate. Environ. Res. 2022, 211, 112971. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Tee, W.T.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Applicability of a novel and highly effective adsorbent derived from industrial palm oil mill sludge for copper sequestration: Central composite design optimisation and adsorption performance evaluation. J. Environ. Chem. Eng. 2021, 9, 105968. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Apiratikul, R.; Chu, K.H. Improved fixed bed models for correlating asymmetric adsorption breakthrough curves. J. Water Process. Eng. 2021, 40, 101810. [Google Scholar] [CrossRef]
- Lai, K.C.; Hiew, B.Y.Z.; Tee, W.T.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Usage of a new macro-hierarchical graphene sponge in batch adsorption and packed column configuration for efficient decontamination of cadmium in aqueous environment. J. Environ. Chem. Eng. 2021, 9, 106057. [Google Scholar] [CrossRef]
- Loo, S.L.; Vásquez, L.; Athanassiou, A.; Fragouli, D. Polymeric Hydrogels—A Promising Platform in Enhancing Water Security for a Sustainable Future. Adv. Mater. Interfaces 2021, 8, 2100580. [Google Scholar] [CrossRef]
- Boateng, E.; Dondapati, J.S.; Thiruppathi, A.R.; Chen, A. Significant enhancement of the electrochemical hydrogen uptake of reduced graphene oxide via boron-doping and decoration with Pd nanoparticles. Int. J. Hydrog. Energy 2020, 45, 28951–28963. [Google Scholar] [CrossRef]
- Qiu, X.; Cai, H.; Fang, X.; Zheng, J. The improved thermal oxidative stability of silicone rubber by incorporating reduced graphene oxide: Impact factors and action mechanism. Polym. Compos. 2018, 39, 1105–1115. [Google Scholar] [CrossRef]
- Ngidi, N.P.D.; Ollengo, M.A.; Nyamori, V.O. Tuning the properties of boron-doped reduced graphene oxide by altering the boron content. New J. Chem. 2020, 44, 16864–16876. [Google Scholar] [CrossRef]
- Fan, X.; Cai, C.; Gao, J.; Han, X.; Li, J. Hydrothermal reduced graphene oxide membranes for dyes removing. Sep. Purif. Technol. 2020, 241, 116730. [Google Scholar] [CrossRef]
- Chintalapudi, K.; Pannem, R.M.R. The effects of Graphene Oxide addition on hydration process, crystal shapes, and microstructural transformation of Ordinary Portland Cement. J. Build. Eng. 2020, 32, 101551. [Google Scholar] [CrossRef]
- Khushbu; Jindal, R. Sodium Alginate and Chitosan Based Amphoteric Nanocomposites Modified with Graphene Oxide and Bentonite as an Efficient Adsorbent for Both Anionic and Cationic Dyes. J. Polym. Environ. 2023, 31, 264–286. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Viji, A.; Balachandran, V.; Babiyana, S.; Narayana, B.; Salian, V.V. FT-IR and FT-Raman investigation, quantum chemical studies, molecular docking study and antimicrobial activity studies on novel bioactive drug of 1-(2,4-Dichlorobenzyl)-3-[2-(3-(4-chlorophenyl)-5-(4-(propan-2-yl)phenyl-4,5-dihydro-1H-pyrazol-1-yl]-4-oxo-4,5-dihydro-1,3-thiazol-5(4H)-ylidence]-2,3-dihydro-1H-indol-2-one. J. Mol. Struct. 2020, 1215, 128244. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Gan, S.Y.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Pan, G.T.; Yang, T.C.K. Adsorptive removal of diclofenac by graphene oxide: Optimization, equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2019, 98, 150–162. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Facile synthesis of xanthan biopolymer integrated 3D hierarchical graphene oxide/titanium dioxide composite for adsorptive lead removal in wastewater. Bioresour. Technol. 2020, 309, 123296. [Google Scholar] [CrossRef]
- Xu, H.; Li, G.; Li, J.; Chen, C.; Ren, X. Interaction of Th(IV) with graphene oxides: Batch experiments, XPS investigation, and modeling. J. Mol. Liq. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Altuntepe, A.; Zan, R. Permanent Boron Doped Graphene with high Homogeneity using Phenylboronic Acid. J. Mol. Struct. 2021, 1230, 129629. [Google Scholar] [CrossRef]
- Sahoo, M.; Sreena, K.P.; Vinayan, B.P.; Ramaprabhu, S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Karri, R.R.; Tanzifi, M.; Tavakkoli Yaraki, M.; Sahu, J.N. Optimization and modeling of methyl orange adsorption onto polyaniline nano-adsorbent through response surface methodology and differential evolution embedded neural network. J. Environ. Manag. 2018, 223, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.; Ngadi, N.; Khairunnisa Awg Zaini, D.; Jusoh, M.; Mohamad, Z.; Arsad, A. Effect of Adsorption Parameter on the Removal of Aspirin Using Tyre Waste Adsorbent. Chem. Eng. Trans. 2019, 72, 157–162. [Google Scholar] [CrossRef]
- Momina; Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef] [PubMed]
- Patel, H. Review on solvent desorption study from exhausted adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar] [CrossRef]
- Abu Alwan, R.; Zhuman, B.; Kumar, M.; Arafat, H.A.; AlNashef, I. Mussel-inspired polydopamine functionalized with ionic liquid as a novel, eco-efficient adsorbent for the selective removal of anionic pollutants from aqueous solutions. Chem. Eng. J. 2023, 454, 140498. [Google Scholar] [CrossRef]









| Run | A (mg) | B (min) | C (ppm) | D (°C) | Ract (%) | Rpred (%) | Residue |
|---|---|---|---|---|---|---|---|
| 1 | 7.5 | 30 | 100 | 50 | 52.36 | 55.87 | −3.510 |
| 2 | 10.0 | 20 | 125 | 60 | 63.66 | 64.00 | −0.336 |
| 3 | 5.0 | 40 | 75 | 40 | 59.96 | 58.71 | 1.240 |
| 4 | 7.5 | 30 | 100 | 50 | 57.15 | 55.87 | 1.280 |
| 5 | 5.0 | 40 | 125 | 60 | 53.32 | 47.83 | 5.490 |
| 6 | 10.0 | 20 | 125 | 40 | 62.64 | 59.58 | 3.070 |
| 7 | 7.5 | 30 | 100 | 30 | 62.80 | 64.60 | −1.800 |
| 8 | 7.5 | 30 | 100 | 50 | 51.78 | 55.87 | −4.090 |
| 9 | 5.0 | 40 | 75 | 60 | 51.27 | 56.67 | −5.390 |
| 10 | 7.5 | 30 | 50 | 50 | 78.60 | 76.95 | 1.650 |
| 11 | 12.5 | 30 | 100 | 50 | 83.19 | 80.96 | 2.220 |
| 12 | 7.5 | 30 | 100 | 50 | 59.04 | 55.87 | 3.170 |
| 13 | 10.0 | 40 | 75 | 60 | 88.08 | 88.01 | 0.064 |
| 14 | 10.0 | 20 | 75 | 60 | 74.72 | 76.60 | −1.880 |
| 15 | 7.5 | 30 | 100 | 50 | 55.60 | 55.87 | −0.271 |
| 16 | 5.0 | 20 | 125 | 40 | 39.84 | 39.00 | 0.845 |
| 17 | 7.5 | 30 | 150 | 50 | 56.23 | 56.47 | −0.233 |
| 18 | 7.5 | 10 | 100 | 50 | 50.62 | 49.11 | 1.510 |
| 19 | 10.0 | 20 | 75 | 40 | 66.64 | 71.22 | −4.580 |
| 20 | 5.0 | 40 | 125 | 40 | 50.38 | 50.83 | −0.446 |
| 21 | 7.5 | 30 | 100 | 50 | 59.29 | 55.87 | 3.420 |
| 22 | 7.5 | 30 | 100 | 70 | 70.19 | 66.97 | 3.210 |
| 23 | 5.0 | 20 | 125 | 60 | 33.16 | 38.01 | −4.850 |
| 24 | 5.0 | 20 | 75 | 40 | 51.76 | 50.80 | 0.958 |
| 25 | 10.0 | 40 | 125 | 40 | 75.48 | 76.92 | −1.440 |
| 26 | 7.5 | 50 | 100 | 50 | 72.27 | 72.36 | −0.090 |
| 27 | 10.0 | 40 | 125 | 60 | 76.05 | 79.33 | −3.280 |
| 28 | 2.5 | 30 | 100 | 50 | 28.23 | 29.04 | −0.806 |
| 29 | 5.0 | 20 | 75 | 60 | 53.11 | 50.76 | 2.350 |
| 30 | 10.0 | 40 | 75 | 40 | 87.18 | 84.65 | 2.530 |
| Model | Equation | Reference |
|---|---|---|
| Thomas | [29,30] | |
| Log Thomas | ||
| Yoon–Nelson | ||
| Log Yoon–Nelson | ||
| Bohart–Adams | ||
| Log Bohart–Adams |
| Element (at%) | GO | BGO | 3D/BGO (Before Adsorption) | 3D/BGO (After Adsorsption) |
|---|---|---|---|---|
| C | 63.64 | 67.02 | 52.71 | 72.52 |
| O | 34.03 | 18.02 | 34.37 | 14.77 |
| B | - | 14.20 | 12.92 | 12.64 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5931.46 | 14 | 423.68 | 28.68 | <0.0001 |
| A | 4044.68 | 1 | 4044.68 | 273.78 | <0.0001 |
| B | 810.80 | 1 | 810.80 | 54.88 | <0.0001 |
| C | 629.48 | 1 | 629.48 | 42.61 | <0.0001 |
| D | 8.47 | 1 | 8.47 | 0.5730 | 0.4608 |
| AB | 30.37 | 1 | 30.37 | 2.06 | 0.1721 |
| AC | 0.0234 | 1 | 0.0234 | 0.0016 | 0.9688 |
| AD | 29.25 | 1 | 29.25 | 1.98 | 0.1798 |
| BC | 15.36 | 1 | 15.36 | 1.04 | 0.3240 |
| BD | 4.04 | 1 | 4.04 | 0.2735 | 0.6086 |
| CD | 0.9126 | 1 | 0.9126 | 0.0618 | 0.8071 |
| A2 | 1.29 | 1 | 1.29 | 0.0875 | 0.7714 |
| B2 | 40.58 | 1 | 40.58 | 2.75 | 0.1182 |
| C2 | 201.50 | 1 | 201.50 | 13.64 | 0.0022 |
| D2 | 168.61 | 1 | 168.61 | 11.41 | 0.0041 |
| Residual | 221.60 | 15 | 14.77 | ||
| Lack of Fit | 169.14 | 10 | 16.91 | 1.61 | 0.3118 |
| Pure Error | 52.46 | 5 | 10.49 | ||
| Cor Total | 6153.06 | 29 | |||
| Std. Dev. | 3.84 | R2 | 0.9640 | ||
| Mean | 60.82 | Adjusted R2 | 0.9304 | ||
| C.V. % | 6.32 | Predicted R2 | 0.8294 | ||
| Adequate Precision | 21.70 |
| Case | Concentration (ppm) | Flowrate (mL/min) | Bed Height (cm) | Dosage (g) | mads (mg) | q (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 50 | 2 | 3.5 | 0.125 | 45.66 | 365.32 |
| 2 | 100 | 2 | 3.5 | 0.125 | 55.90 | 447.24 |
| 3 | 50 | 3 | 3.5 | 0.125 | 51.73 | 413.83 |
| 4 | 50 | 2 | 3 | 0.1 | 30.86 | 308.57 |
| Condition | Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bohart–Adams | Log Bohart–Adams | ||||||||||
| z (cm) | C0 (ppm) | Q (mL/min) | Dosage (g) | kBA (mL/mg·min) | N0 (mg/mL) | R2 | RRMSE | kBA (mL/mg·min) | N0 (mg/mL) | R2 | RRMSE |
| 3.5 | 50 | 2 | 0.125 | 0.092 | 16.041 | 0.961 | 0.071 | 1.614 | 12.435 | 0.992 | 0.033 |
| 3.5 | 100 | 2 | 0.125 | 0.064 | 15.086 | 0.927 | 0.085 | 1.127 | 10.217 | 0.991 | 0.03 |
| 3.5 | 50 | 3 | 0.125 | 0.139 | 18.393 | 0.989 | 0.041 | 2.062 | 15.503 | 0.987 | 0.987 |
| 3 | 50 | 2 | 0.1 | 0.118 | 11.695 | 0.964 | 0.066 | 1.367 | 8.379 | 0.982 | 0.046 |
| Thomas | Log Thomas | ||||||||||
| kTH (mL/mg·min) | q0 (mg/g) | kTH (mL/mg·min) | q0 (mg/g) | ||||||||
| 3.5 | 50 | 2 | 0.125 | 0.092 | 352.76 | 1.614 | 273.46 | ||||
| 3.5 | 100 | 2 | 0.125 | 0.064 | 331.77 | 1.127 | 224.68 | ||||
| 3.5 | 50 | 3 | 0.125 | 0.139 | 404.48 | 2.062 | 340.94 | ||||
| 3 | 50 | 2 | 0.1 | 0.118 | 275.55 | 1.367 | 197.42 | ||||
| Yoon–Nelson | Log Yoon–Nelson | ||||||||||
| KYN (min−1) | τ (min) | KYN (min−1) | τ (min) | ||||||||
| 3.5 | 50 | 2 | 0.125 | 0.005 | 440.95 | 1.614 | 341.83 | ||||
| 3.5 | 100 | 2 | 0.125 | 0.006 | 207.35 | 1.127 | 140.43 | ||||
| 3.5 | 50 | 3 | 0.125 | 0.007 | 337.06 | 2.062 | 284.11 | ||||
| 3 | 50 | 2 | 0.1 | 0.006 | 275.55 | 1.367 | 197.42 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tee, W.T.; Chua, J.; Yong, J.E.; Hiew, B.Y.Z.; Gan, S.; Lee, L.Y. Remediation of Amitriptyline Pharmaceutical Wastewater by Heteroatom-Doped Graphene Oxide: Process Optimization and Packed-Bed Studies. Separations 2023, 10, 392. https://doi.org/10.3390/separations10070392
Tee WT, Chua J, Yong JE, Hiew BYZ, Gan S, Lee LY. Remediation of Amitriptyline Pharmaceutical Wastewater by Heteroatom-Doped Graphene Oxide: Process Optimization and Packed-Bed Studies. Separations. 2023; 10(7):392. https://doi.org/10.3390/separations10070392
Chicago/Turabian StyleTee, Wan Ting, Jasmine Chua, Jia En Yong, Billie Yan Zhang Hiew, Suyin Gan, and Lai Yee Lee. 2023. "Remediation of Amitriptyline Pharmaceutical Wastewater by Heteroatom-Doped Graphene Oxide: Process Optimization and Packed-Bed Studies" Separations 10, no. 7: 392. https://doi.org/10.3390/separations10070392
APA StyleTee, W. T., Chua, J., Yong, J. E., Hiew, B. Y. Z., Gan, S., & Lee, L. Y. (2023). Remediation of Amitriptyline Pharmaceutical Wastewater by Heteroatom-Doped Graphene Oxide: Process Optimization and Packed-Bed Studies. Separations, 10(7), 392. https://doi.org/10.3390/separations10070392