Fast Procedure for Removing Silver Species in Waters Using a Simple Magnetic Nanomaterial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
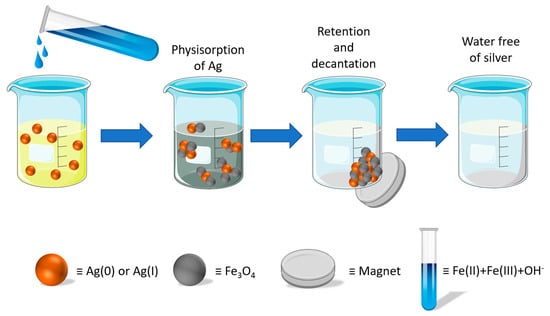
2.2. In Situ Preparation of Fe3O4 Nanoparticles and Silver Removal Procedure
3. Results
3.1. Characterization of Fe3O4
3.2. Effect of Fe3O4 Precursors Volume
3.3. Effect of Contact Time
3.4. Study of the Effect of the Temperature and Adsorption Isotherms
3.5. Mechanism of Adsorption
3.6. Comparison with Other Removal Methods
3.7. Study of Competition with Other Ions Present in Water. Application to Real Water Samples and Reuse of Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patnaik, P. Emerging Pollutants: Nanomaterials. In Handbook of Environmental Analysis. Chemical Pollutants in Air Water, Soil and Soil Wastes, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Salthammer, T. Emerging indoor pollutants. Int. J. Hyg. Environ. Health 2020, 224, 113423. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; Solana-Gonzalez, R. Magnetic core-modified silver nanoparticles for ibuprofen removal: An emerging pollutant in waters. Sci. Rep. 2020, 10, 18288. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Sandoval, M.J.; Caravaca, M.; López-García, I.; Hernández-Córdoba, M.; Vicente-Martínez, Y. Complete and simultaneous removal of ionic silver and silver nanoparticles by using an ionic liquid supported on a magnetic nanoparticle core. Environ. Res. 2022, 214, 113943. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Purushothaman, B.; Rangasamy, S.; Song, J.M. Investigating the versatility of multifunctional silver nanoparticles: Preparation and inspection of their potential as wound treatment agents. Int. Nano Lett. 2016, 6, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Chapter 14—Antimicrobial activity of silver nanoparticles. In Nanoparticles in Pharmacotherapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 461–484. [Google Scholar] [CrossRef]
- Maurer, L.L.; Meyer, J.N. A systematic review of evidence for silver nanoparticle-induced mitochondrial toxicity. Environ. Sci. Nano 2016, 3, 311–322. [Google Scholar] [CrossRef]
- López-García, I.; Vicente-Martínez, Y.; Hernández-Córdoba, M. Speciation of silver nanoparticles and Ag(I) species using cloud point extraction followed by electrothermal atomic absorption spectrometry. Spectroc. Acta Part B-Atom. Spectr. 2014, 101, 93–97. [Google Scholar] [CrossRef]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbuehler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Edit. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; Gomez-Gomez, B.; Eugenia Leon-Gonzalez, M.; Madrid, Y. Characterization of AgNPs and AuNPs in sewage sludge by single particle inductively coupled plasma-mass spectrometry. Talanta 2022, 238, 123033. [Google Scholar] [CrossRef]
- Montoro, L.A.; de Freitas, R.P.; Silva, H.; Sinisterra, R.D.; dos Santos, E.N. Disinfectant products to face the pandemic COVID-19. Rev. Virtual de Quimica 2020, 12, 1114–1128. [Google Scholar] [CrossRef]
- Eggers, M.; Baumann, A.; Lilienthal, N.; Steinmann, E.; Steinmann, J.; Hubner, N.-O.; Rabenau, H.F.; Weinheimer, V.; Schwebke, I. Disinfectants during the COVID-19 pandemic: A challenge. Bundesgesundheitsbla 2022, 65, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zuarez-Chamba, M.; Rajendran, S.; Herrera-Robledo, M.; Priya, A.K.; Navas-Cardenas, C. Bi-based photocatalysts for bacterial inactivation in water: Inactivation mechanisms, challenges, and strategies to improve the photocatalytic activity. Environ. Res. 2022, 209, 112834. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Rajendran, S.; Kumar, P.S.; Priya, A.K.; Gracia, F.; Habila, M.A.; Saravanakumar, K. Visible light stimulated binary nanostructure and defect enriched TiO2-SnO2 for photocatalysis and antibacterial activity. Mater. Lett. 2022, 316, 131998. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Rajendran, S.; Priya, A.K.; Durgalakshmi, D.; Vo, D.-V.N.; Cornejo-Ponce, L.; Gracia, F.; Soto-Moscoso, M. Photocatalytic degradation of 2,4-dichlorophenol using bio-green assisted TiO2-CeO2 nanocomposite system. Environ. Res. 2021, 195, 110852. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhao, Y.X.; Li, X.F.; Ren, Y.P. Performance evaluation of a microfiltration-osmotic membrane bioreactor (MF-OMBR) during removing silver nanoparticles from simulated wastewater. Chem. Eng. J. 2017, 313, 171–178. [Google Scholar] [CrossRef]
- Ahmed, T.; Bhatti, Z.A.; Maqbool, F.; Mahmood, Q.; Faridullah; Qayyum, S.; Mushtaq, N. A comparative study of synthetic and natural coagulants for silver nanoparticles removal from wastewater. Desalin. Water Treat. 2016, 57, 18718–18723. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Tang, T.; Yuan, Z.; Yu, C.-P. Removal of silver nanoparticles by coagulation processes. J. Hazard. Mater. 2013, 261, 414–420. [Google Scholar] [CrossRef]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Adsorptive removal of silver nanoparticles (SNPs) from aqueous solution by Aeromonas punctata and its adsorption isotherm and kinetics. Colloid Surf. B 2012, 92, 156–160. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Mallampati, R.; Sriramulu, D.; Dsouza, R.F.; Valiyaveettil, S. PVA/Gluten hybrid nanofibers for removal of nanoparticles from water. ACS Sustain. Chem. Eng. 2014, 2, 1014–1021. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, S.; Urper Bayram, G.M.; Khati, P.; Simsek, H. Developments and application of chitosan-based adsorbents for wastewater treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Liu, H.; He, Z.; Show, P.L.; Vasseghian, Y.; Wang, C. Biochar/layered double hydroxides composites as catalysts for treatment of organic wastewater by advanced oxidation processes: A review. Environ. Res. 2023, 116534. [Google Scholar] [CrossRef] [PubMed]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of fruit waste derived bio-adsorbents for wastewater treatment: A review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Ishihara, M.; Vinh Quang, N.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [Green Version]
- Dzhimak, S.S.; Malyshko, V.V.; Goryachko, A.I.; Sokolov, M.E.; Moiseev, A.V.; Basov, A.A. Adsorption of silver nanoparticles on mono- and polyfilament fibers. Nanotechnol. Russ. 2019, 14, 48–54. [Google Scholar] [CrossRef]
- Polowczyk, I.; Kozlecki, T.; Bastrzyk, A. Adsorption of Silver Nanoparticles on Glass Beads Surface. Adosrpt. Sci. Technol. 2015, 33, 731–737. [Google Scholar] [CrossRef]
- Oh, S.Y.; Sung, H.K.; Park, C.; Kim, Y. Biosorptive removal of bare-, citrate-, and PVP-coated silver nanoparticles from aqueous solution by activated sludge. J. Ind. Eng. Chem. 2015, 25, 51–55. [Google Scholar] [CrossRef]
- Gicheva, G.; Yordanov, G. Removal of citrate-coated silver nanoparticles from aqueous dispersions by using activated carbon. Colloid Surf. A 2013, 431, 51–59. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Manoochehri, M.; Taromi, F.A. Fabrication of a novel magnetic metal-organic framework functionalized with 2-aminothiophenol for preconcentration of trace silver amounts in water and wastewater. RSC Adv. 2021, 11, 13867–13875. [Google Scholar] [CrossRef]
- Sim, J.H.; Umh, H.N.; Shin, H.H.; Sung, H.K.; Oh, S.Y.; Lee, B.-C.; Rengaraj, S.; Kim, Y. Comparison of adsorptive features between silver ion and silver nanoparticles on nanoporous materials. J. Ind. Eng. Chem. 2014, 20, 2864–2869. [Google Scholar] [CrossRef]
- Islam, M.A.; Parvin, M.I.; Dada, T.K.; Kumar, R.; Antunes, E. Silver adsorption on biochar produced from spent coffee grounds: Validation by kinetic and isothermal modelling. Biomass Convers. Biorefin. 2022, 1–15. [Google Scholar] [CrossRef]
- Ma, L.-y.; Li, Q.-y.; Yu, X.; Jiang, M.; Xu, L. Recent developments in the removal of metal-based engineered nanoparticles from the aquatic environments by adsorption. Chemosphere 2022, 291, 133089. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Fulazzaky, M.A.; Salmiati, S.; Kueh, A.B.H.; Fulazzaky, M.; Salim, M.R. Silver nanoparticles adsorption by the synthetic and natural adsorbent materials: An exclusive review. Nanotechnol. Environ. Eng. 2020, 5, 1. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A. Simultaneous adsorption of mercury species from aquatic environments using magnetic nanoparticles coated with nanomeric silver functionalized with L-Cysteine. Chemosphere 2021, 282, 131128. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, Y.; Priya, A.K. Removal of heavy metal ion concentrations from the wastewater using tobacco leaves coated with iron oxide nanoparticles. Int. J. Environ. Sci. Technol. 2022, 19, 2721–2736. [Google Scholar] [CrossRef]
- Naeem, H.; Tofil, H.M.; Soliman, M.; Hai, A.; Zaidi, S.H.H.; Kizilbash, N.; Alruwaili, D.; Ajmal, M.; Siddiq, M. Reduced graphene oxide-zinc sulfide nanocomposite decorated with silver nanoparticles for wastewater treatment by adsorption, photocatalysis and antimicrobial action. Molecules 2023, 28, 926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Huang, M.-R.; Li, S.-X. Facile synthesis of poly(1,8-diaminonaphthalene) microparticles with a very high silver-ion adsorbability by a chemical oxidative polymerization. Acta Mater. 2004, 52, 5363–5374. [Google Scholar] [CrossRef]
- Jintakosol, T.; Nitayaphat, W. Adsorption of silver (I) from aqueous solution using chitosan/montmorillonite composite beads. Mater. Res.-Ibero-Am. J. 2016, 19, 1114–1121. [Google Scholar] [CrossRef]
- Li, X.-G.; Ma, X.-L.; Sun, J.; Huang, M.-R. Powerful reactive sorption of silver(i) and mercury(ii) onto poly(o-phenylenediamine) microparticles. Langmuir 2009, 25, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Pongkitdachoti, U.; Unob, F. Simultaneous adsorption of silver nanoparticles and silver ions on large pore mesoporous silica. J. Environ. Chem. Eng. 2018, 6, 596–603. [Google Scholar] [CrossRef]
- Cundeva, K.; Stafilov, T. Flame atomic absorption spectrometric determination of zinc after colloid precipitate flotation with hydrated iron(III) oxide and iron(III) tetramethylenedithiocarbamate as collectors. Talanta 1997, 44, 451–456. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Zhao, Y.; Guan, L. Direct synthesis of iron oxide nanoparticles on an iron current collector as binder-free anode materials for lithium-ion batteries. Mater. Lett. 2012, 81, 105–107. [Google Scholar] [CrossRef]
- Zendelovska, D.; Cundeva, K.; Stafilov, T. Applicability of hydrated iron(III) oxide and dithiocarbamates as colloid collectors for flotation preconcentration of manganese in traces before its ETAAS determination. Microchim. Acta 2000, 135, 55–61. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; Martin-Pereira, M.A.; del Carmen García-Onsurbe, M. Adsorption studies on magnetic nanoparticles functionalized with silver to remove nitrates from waters. Water 2021, 13, 1757. [Google Scholar] [CrossRef]
- López-García, I.; Vicente-Martínez, Y.; Hernández-Córdoba, M. Determination of ultratraces of mercury species using separation with magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. J. Anal. Atom. Spectrom. 2015, 30, 1980–1987. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Daniele, S.; El Nemr, A.; Abouelanwar, M.E.; Morcos, B.M. Recovery of silver and gold quantum dots from wastewater via coagulative adsorption onto CoFe2O4 based magnetic covalent-organic framework to generate efficient nanocatalysts for degradation of doxorubicin drug. J. Water Process. Eng. 2023, 51, 103409. [Google Scholar] [CrossRef]
- Neyestani, M.R.; Shemirani, F.; Mozaffari, S.; Alvand, M. A magnetized graphene oxide modified with 2-mercaptobenzothiazole as a selective nanosorbent for magnetic solid phase extraction of gold(III), palladium(II) and silver(I). Microchim. Acta 2017, 184, 2871–2879. [Google Scholar] [CrossRef]
- Lan Huong, P.T.; Tu, N.; Lan, H.; Thang, L.H.; Van Quy, N.; Tuan, P.A.; Dinh, N.X.; Phan, V.N.; Le, A.-T. Functional manganese ferrite/graphene oxide nanocomposites: Effects of graphene oxide on the adsorption mechanisms of organic MB dye and inorganic As(v) ions from aqueous solution. RSC Adv. 2018, 8, 12376–12389. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Dadfarnia, S.; Shabani, A.M.H. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tolessa, T.; Zhou, X.-X.; Amde, M.; Liu, J.-F. Development of reusable magnetic chitosan microspheres adsorbent for selective extraction of trace level silver nanoparticles in environmental waters prior to ICP-MS analysis. Talanta 2017, 169, 91–97. [Google Scholar] [CrossRef]
- Zhao, B.; He, M.; Chen, B.; Hu, B. Ligand-assisted magnetic solid phase extraction for fast speciation of silver nanoparticles and silver ions in environmental water. Talanta 2018, 183, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Mashhadizadeh, M.H.; Amoli-Diva, M.; Shapouri, M.R.; Afruzi, H. Solid phase extraction of trace amounts of silver, cadmium, copper, mercury, and lead in various food samples based on ethylene glycol bis-mercaptoacetate modified 3-(trimethoxysilyl)-1-propanethiol coated Fe3O4 nanoparticles. Food Chem. 2014, 151, 300–305. [Google Scholar] [CrossRef]
- Odio, O.F.; Lartundo-Rojas, L.; Santiago-Jacinto, P.; Martínez, R.; Reguera, E. Sorption of gold by naked and thiol-capped magnetite nanoparticles: An XPS approach. J. Phys. Chem. C 2014, 118, 2776–2791. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y. Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver(I), gold(III), copper(II) and palladium(II). Microchim. Acta 2014, 181, 543–551. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, K.; Chen, Q.; Wang, A.; Chen, W. Preparation of magnetic ferrite by optimizing the synthetic pH and its application for the removal of Cd(II) from Cd-NH3-H2O system. J. Mol. Liq. 2018, 264, 215–222. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Caravaca, M.; Vicente-Martínez, Y.; Soto-Meca, A.; Angulo-Gonzalez, E. Total removal of amoxicillin from water using magnetic core nanoparticles functionalized with silver. Environ. Res. 2022, 211, 113091. [Google Scholar] [CrossRef]
- Lloyd, G.E. Atomic-number and crystallographic contrast images with the sem—A review of backscattered electron techniques. Minerl Mag. 1987, 51, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, E.; Torres Deluigi, M.; Castellano, G. Mean atomic number quantitative assessment in backscattered electron imaging. Microsc. Microanal. 2012, 18, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alardhi, S.; Alrubaye, J.; Albayati, T. Adsorption of Methyl Green dye onto MCM-41: Equilibrium, kinetics and thermodynamic studies. Desalin. Water Treat. 2020, 179, 323–331. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and chromium adsorption from water using L-cysteine functionalized magnetite (Fe3O4) nanoparticles. Sci. Rep. 2017, 7, 7672. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Li, Y.; Huang, G.; Yang, C.; Chen, C.; Zhou, T.; Zhao, Y.; Ma, J. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 2020, 260, 127650. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Hakim, L.F.; Blackson, J.H.; Weimer, A.W. Modification of interparticle forces for nanoparticles using atomic layer deposition. Chem. Eng. Sci. 2007, 62, 6199–6211. [Google Scholar] [CrossRef]
- Qin, Y.; Fichthorn, K.A. Molecular dynamics simulation of the forces between colloidal nanoparticles in n-decane solvent. J. Chem. Phys. 2007, 127, 144911. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ali, S.M.; El-Dek, S.I.; Galal, A. Magnetite–hematite nanoparticles prepared by green methods for heavy metal ions removal from water. Mater. Sci. Eng. B 2013, 178, 744–751. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 8, 720654. [Google Scholar] [CrossRef] [Green Version]
- Salih, H.; Badawy, A.; Tolaymat, T.; Patterson, C.; Salih, H. Removal of stabilized silver nanoparticles from surface water by conventional treatment processes. ANP 2019, 8, 21–35. [Google Scholar] [CrossRef]
- Liu, J.-f.; Chao, J.-b.; Liu, R.; Tan, Z.-q.; Yin, Y.-g.; Wu, Y.; Jiang, G.-b. Cloud point extraction as an advantageous preconcentration approach for analysis of trace silver nanoparticles in environmental waters. Anal. Chem. 2009, 81, 6496–6502. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Chen, B.B.; Hu, B. Ultra-trace determination of gold nanoparticles in environmental water by surfactant assisted dispersive liquid liquid microextraction coupled with electrothermal vaporization-inductively coupled plasma-mass spectrometry. Spectroc. Acta Part B-Atom. Spectr. 2016, 122, 94–102. [Google Scholar] [CrossRef]
- Krizkova, S.; Kryštofová, O.; Trnkova, L.; Hubalek, J.; Adam, V.; Beklova, M.; Horna, A.; Havel, L.; Kizek, R. Silver(I) ions ultrasensitive detection at carbon electrodes-analysis of waters, tobacco cells and fish tissues. Sensors 2009, 9, 6934–6950. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gunawan, P.; Jiang, R.; Leong, S.S.J.; Wang, K.; Xu, R. Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions. J. Hazard. Mater. 2011, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Zhang, M.; Helleur, R.; Zhang, Y. Ion-imprinted chitosan gel beads for selective adsorption of Ag+ from aqueous solutions. Carbohydr. Polym. 2015, 130, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, X.; Pan, Q.; Ao, Y.; Du, J.; Zhai, M.; Zhao, L. Performance and mechanism of selective adsorption of silver to L-cysteine functionalized cellulose microsphere. Cellulose 2020, 27, 3249–3261. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Alandis, N.M.; Mekhamer, W.; Aldayel, O.; Hefne, J.A.A.; Alam, M. Adsorptive applications of montmorillonite clay for the removal of Ag(I) and Cu(II) from aqueous medium. J. Chem. 2019, 2019, 7129014. [Google Scholar] [CrossRef] [Green Version]
- Sprynskyy, M.; Sokol, H.; Rafińska, K.; Brzozowska, W.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Preparation of AgNPs/saponite nanocomposites without reduction agents and study of its antibacterial activity. Colloid Surf. B 2019, 180, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Cantuaria, M.L.; de Almeida Neto, A.F.; Nascimento, E.S.; Vieira, M.G.A. Adsorption of silver from aqueous solution onto pre-treated bentonite clay: Complete batch system evaluation. J. Clean. Prod. 2016, 112, 1112–1121. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K.; Sokołowska, Z.; Kercheva, M.; Dimitrov, E. Purification of aqueous media by biochars: Feedstock type effect on silver nanoparticles removal. Molecules 2020, 25, 2930. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Zhao, Y.; Wang, N.; Bai, J.; Feng, K.; Zhou, Y.; Chen, W.; Wen, F.; Wang, S.; et al. Pyrogenic temperature affects the particle size of biochar-supported nanoscaled zero valent iron (nZVI) and its silver removal capacity. Chem. Speciat. Bioavailab. 2017, 29, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.-x.; Li, Y.-j.; Liu, J.-f. Highly efficient removal of silver-containing nanoparticles in waters by aged iron oxide magnetic particles. ACS Sustain. Chem. Eng. 2017, 5, 5468–5476. [Google Scholar] [CrossRef]
- Conde-González, J.E.; Peña-Méndez, E.M.; Rybáková, S.; Pasán, J.; Ruiz-Pérez, C.; Havel, J. Adsorption of silver nanoparticles from aqueous solution on copper-based metal organic frameworks (HKUST-1). Chemosphere 2016, 150, 659–666. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Wang, Y.-H.; Huang, Y.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. Fe3O4–Mg(OH)2 nanocomposite as a scavenger for silver nanoparticles: Rational design, facile synthesis, and enhanced performance. Environ. Res. 2022, 212, 113292. [Google Scholar] [CrossRef]
- Hassan, D.; Farghali, M. Adsorption of silver nanoparticles from aqueous solution by multiwalled carbon nanotubes. ANP 2017, 6, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q. Removal and reuse of Ag nanoparticles by magnetic polyaniline/Fe3O4 nanofibers. J. Mater. Sci. 2018, 53, 8901–8908. [Google Scholar] [CrossRef]








| Parameter | |||
|---|---|---|---|
| Wavelength, nm | 328.068 | ||
| Slit, nm | 0.7 | ||
| Atomizer | Transversal with platform of L’Vov | ||
| Background correction | Efecto Zeeman | ||
| Injected volume, µL | 10 | ||
| Chemical modifier | 10 µL of Pd(II) (250 mg L−1) | ||
| Heating program | |||
| Step | Temperature, °C | Ramp, °C s−1 | Hold, s |
| 1: Dry | 110 | 10 | 20 |
| 2: Dry | 130 | 9 | 10 |
| 3: Ashing | 400 | 20 | 20 |
| 4: Atomization a,b | 1700 | 1500 | 5 |
| 5: Cleaning | 2450 | 500 | 3 |
| Model | Parameter | Adsorbate | |
|---|---|---|---|
| Ag(0) | Ag(I) | ||
| Pseudo-first-order | k1, min−1 | 0.028 | 0.031 |
| qe, mg∙g−1 | 18.75 | 19.21 | |
| R2 | 0.8842 | 0.8291 | |
| Pseudo-second-order | k2, min−1 | 0.082 | 0.098 |
| qe, mg∙g−1 | 19.02 | 19.31 | |
| R2 | 0.9942 | 0.9975 | |
| Langmuir Model | Freundlich Model | |||
|---|---|---|---|---|
| T, °C | ||||
| 24 | 0.9997 | 5.58 × 10–9 | 0.9991 | 1.64 × 10–5 |
| 30 | 0.99986 | 7.23 × 10–9 | 0.09981 | 3.94 × 10–5 |
| 50 | 0.9999 | 1.33 × 10–9 | 0.9993 | 2.59 × 10–6 |
| Type of Adsorbents | Adsorbent | Adsorption Capacity (mg g−1) | pH | Initial Concentration of Silver (mg L−1) | Contact Time | Ref. |
|---|---|---|---|---|---|---|
| Activate carbon | Norit® CA1 | 65 | 3–9 | 50–105 | 12 h | [34] |
| Colloidal carbon nanospheres | 152 | 3–9 | 0.1–202 | 6 min–32 h | [79] | |
| Biowaste materials | Chitosan | 42 | 6 | 50 | 1–96 h | [80] |
| Ion-imprinted chitosan gel beads | 89.2 | 5 | 353 | 1–48 | [81] | |
| Modified cellulose | L-cysteine functionalized | 66.7 | 6.9 | 160 | 1–10 h | [82] |
| Nanocrystals | 19.8 | 6.6 | 108 | 2 h | [83] | |
| Clays | Montmorillonite | 63.3 | 6 | 200 | 1–5 h | [84] |
| Saponite | 48.3 | 4–8 | 2000 | 5 h | [85] | |
| Bentonite | 61.5 | 6–7 | 50–200 | 400 | [86] | |
| Biochars | Vineyard | 88.9 | 5 | 50 | 70 min | [87] |
| zero valent iron (nZVI) | 500–700 | - | 25 | 24 h | [88] | |
| Spent coffee ground | 49.0 | 6–8 | 50 | 10 h | [37] | |
| Synthetic materials | Aged iron oxide magnetic particles | 20–63 | 6.2 | 100 | 90 min | [89] |
| Metal organic frameworks | 90 | 6–7 | 5 | 10 min | [90] | |
| Nanoporous silica | 396 | 6–7 | 5–200 | 24 h | [36] | |
| Fe3O4-Mg(OH)2 | 476 | 5–11 | 54 | 24 h | [91] | |
| Fe3O4-IL | 103 | 7–9 | 0.2 | 14 min | [5] | |
| Multi-walled carbon nanotubes | 43.2 | 7 | 132 | 240 | [92] | |
| Polyaniline Fe3O4 nanofibers | 12.6 | 5–7 | 0.05 | 120 | [93] | |
| Fe3O4 synthetized in situ | 142–135 | 9 | 0.1 | 5 | This work |
| Water Sample | [Ag] Found | Total [Ag] Added a, µg L−1 | Removal Efficiency b, % |
|---|---|---|---|
| River 1 | ≤LOD | 200 | 98.2 ± 2.3 |
| River 2 | ≤LOD | 200 | 97.1 ± 2.7 |
| River 3 | ≤LOD | 200 | 98.4 ± 3.3 |
| Drinking water 1 | ≤LOD | 200 | 99.2 ± 2.1 |
| Drinking water 2 | ≤LOD | 200 | 99.1 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Martínez, Y.; Ruiz-Mendieta, M.; Caravaca-Garratón, M.; Hernández-Córdoba, M.; López-García, I. Fast Procedure for Removing Silver Species in Waters Using a Simple Magnetic Nanomaterial. Separations 2023, 10, 398. https://doi.org/10.3390/separations10070398
Vicente-Martínez Y, Ruiz-Mendieta M, Caravaca-Garratón M, Hernández-Córdoba M, López-García I. Fast Procedure for Removing Silver Species in Waters Using a Simple Magnetic Nanomaterial. Separations. 2023; 10(7):398. https://doi.org/10.3390/separations10070398
Chicago/Turabian StyleVicente-Martínez, Yésica, Moisés Ruiz-Mendieta, Manuel Caravaca-Garratón, Manuel Hernández-Córdoba, and Ignacio López-García. 2023. "Fast Procedure for Removing Silver Species in Waters Using a Simple Magnetic Nanomaterial" Separations 10, no. 7: 398. https://doi.org/10.3390/separations10070398
APA StyleVicente-Martínez, Y., Ruiz-Mendieta, M., Caravaca-Garratón, M., Hernández-Córdoba, M., & López-García, I. (2023). Fast Procedure for Removing Silver Species in Waters Using a Simple Magnetic Nanomaterial. Separations, 10(7), 398. https://doi.org/10.3390/separations10070398