Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues
Abstract
:1. Introduction
1.1. The Role of Climate Change in the Necessity of Irrigation
1.2. The Role of Irrigation Systems
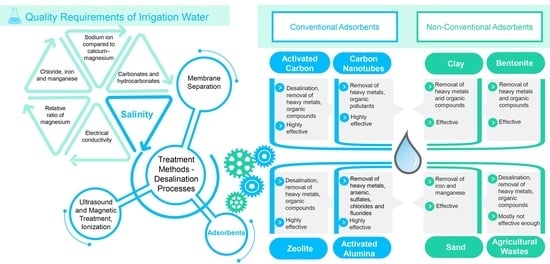
2. Overview of the Requirements for Irrigation Water
2.1. Legal Requirements
2.2. What Water Sources Can We Work with?
2.3. Quality Requirements of Irrigation Water
- salinity,
- indicators expressing the effect of hydrocarbons and carbonates,
- the amount of sodium ions compared to calcium–magnesium ions,
- the relative ratio of magnesium,
- electrical conductivity,
- chloride, iron and manganese content [26].
2.3.1. pH
2.3.2. Salinity
2.3.3. The Amount of Sodium Ions Compared to Calcium-Magnesium Ions
2.3.4. Manganese Content
3. Physical Structure of Irrigation Systems and the Arising Problems
3.1. Surface Irrigation Systems
3.1.1. Sprinkler Irrigation
3.1.2. Drip Irrigation
3.2. General Structure of Irrigation Systems
3.3. Clogging
3.3.1. Physical Clogging
3.3.2. Chemical Clogging
3.3.3. Biological Clogging
4. Overview of Current Irrigation Water Treatment Methods
4.1. Overview of Contaminants
4.2. Biological Treatment
4.3. Desalination Processes
4.3.1. Ultrasound Treatment
4.3.2. Magnetic Treatment
4.3.3. Ionization
4.3.4. Membrane Separation
4.4. Effects of Salinity
4.5. Adsorbents in Irrigation Water Treatment
5. Overview of the Most Important, Easily Available Adsorbents Based on Their Effectiveness
5.1. Conventional Adsorbents
5.1.1. Activated Carbon
5.1.2. Carbon Nanotubes
5.1.3. Zeolite
5.1.4. Activated Alumina
5.2. Non-Conventional Adsorbents
5.2.1. Clay
5.2.2. Bentonite
5.2.3. Sand
5.2.4. Agricultural Wastes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate Change and Weather Extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Tabari, H. Climate Change Impact on Flood and Extreme Precipitation Increases with Water Availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef] [PubMed]
- Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; 2019; pp. 1–864.
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Khan, S.; Tariq, R.; Yuanlai, C.; Blackwell, J. Can Irrigation Be Sustainable? Agric. Water Manag. 2006, 80, 87–99. [Google Scholar] [CrossRef]
- Hagan, R.M. Water-Soil-Plant Relations. Calif. Agric. 1957, 11, 9–12. [Google Scholar]
- Ampim, P.A.Y.; Obeng, E.; Olvera-Gonzalez, E. Indoor Vegetable Production: An Alternative Approach to Increasing Cultivation. Plants 2022, 11, 2843. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, M.E.; Cruz-Cano, R.; Solaiman, S.; Ammons, S.; Allard, S.M.; Sapkota, A.R.; Micallef, S.A.; Goldstein, R.E.R. Impact of Irrigation Water Type and Sampling Frequency on Microbial Water Quality Profiles Required for Compliance with U.S. Food Safety Modernization Act Produce Safety Rule Standards. Environ. Res. 2022, 205, 112480. [Google Scholar] [CrossRef]
- European Council. 91/271/EEC Directive Concerning Urban Waste Water Treatment. Off. J. Eur. Communities II 1991, 135, 40–52. [Google Scholar]
- Council of European Communities. 98/83/EC Directive on the Quality of Water Intended for Human Consumption. Off. J. Eur. Communities 1998, 330, 32–54. [Google Scholar] [CrossRef]
- Act No. LIII of 1995 on the General Rules of Environmental Protection; Budapest, Hungary, 1995.
- Act No. LIII of 1996 on Nature Conservation; Budapest, Hungary, 1996.
- Act No. CLXXXV of 2012 on Waste; Budapest, Hungary, 2012.
- European Parliament; European Council. 2007/60/EC Directive on the Assessment and Management of Flood Risks. Off. J. Eur. Union 2007, 288, 27–34. [Google Scholar]
- European Parliament; European Council. 2000/60/EC Directive Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Communities 2000, 1–72. [Google Scholar]
- Decree of the Ministry of Agriculture No. 90/2008. (VII. 18.) on Detailed Rules for Drawing Up the Soil Protection Plan. Budapest, Hungary, 2008. Available online: https://www.ecolex.org/details/legislation/decree-no-90-of-2008-vii-18-fvm-of-the-ministry-of-agriculture-and-rural-development-laying-down-detailed-rules-of-elaboration-of-soil-conservation-plans-lex-faoc124169/ (accessed on 24 July 2023).
- MSZ-10-640:1989 Water Management. Water Quality. Requirements for the Quality of Irrigation Water (Hungarian Standard). Budapest, Hungary, 1989. Available online: https://oszkdk.oszk.hu/storage/00/02/38/12/dd/1/Alkalmazkod___v__zgazd__lkod__s_ABSZTRAKT.pdf (accessed on 24 July 2023).
- Act No. CXIII on Farming Irrigation; Budapest, Hungary, 2019.
- Paudel, K.P.; Pandit, M.; Roger, H. Irrigation Water Sources and Irrigation Application Methods Used by U.S. Plant Nursery Producers. Water Resour. Res. Res. 2016, 52, 698–712. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Zhang, J.; Yang, Q.; Li, G.; Zhang, D. Impacts of Irrigation Water Sources and Geochemical Conditions on Vertical Distribution of Pharmaceutical and Personal Care Products (PPCPs) in the Vadose Zone Soils. Sci. Total Environ. 2018, 626, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation Water Quality—A Contemporary Perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Stefanovics, P.; Filep, G.; Füleky, G. Talajtan; Mezőgazda Kiadó: Budapest, Hungary, 2010. [Google Scholar]
- Darab, K.; Ferencz, K. Öntözött Területek Talajtérképezése; Országos Mezőgazdasági Minőségvizsgáló Intézet: Budapest, Hungary, 1969. [Google Scholar]
- George, P.R. Agricultural Water Quality Criteria: Irrigation Aspects; Department of Agriculture: Merredin, Australia, 1983. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin, Germany, 2018; ISBN 9783319961897. [Google Scholar]
- Zhao, D.; Hao, Z.; Wang, J.; Tao, J. Effects of PH in Irrigation Water on Plant Growth and Flower Quality in Herbaceous Peony (Paeonia lactiflora Pall.). Sci. Hortic. 2013, 154, 45–53. [Google Scholar] [CrossRef]
- Guimarães, J. de J.; Sousa, F.G.G. de; Román, R.M.S.; Dal Pai, A.; Rodrigues, S.A.; Sarnighausen, V.C.R. Effect of Irrigation Water PH on the Agronomic Development of Hops in Protected Cultivation. Agric. Water Manag. 2021, 253, 106924. [Google Scholar] [CrossRef]
- Adamu, G.K. Quality of Irrigation Water and Soil Characteristics of Watari Irrigation Project. Am. J. Eng. Res. 2013, 02, 59–68. [Google Scholar]
- Seilsepour, M.; Rashidi, M.; Khabbaz, B.G. Prediction of Soil Exchangeable Sodium Ratio Based on Soil Sodium Adsorption Ratio. World Acad. Sci. Eng. Technol. 2010, 46, 255–257. [Google Scholar]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Yuan, C.; Feng, S.; Huo, Z.; Ji, Q. Effects of Deficit Irrigation with Saline Water on Soil Water-Salt Distribution and Water Use Efficiency of Maize for Seed Production in Arid Northwest China. Agric. Water Manag. 2019, 212, 424–432. [Google Scholar] [CrossRef]
- Ajay, S. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef]
- Smith, C.J.; Oster, J.D.; Sposito, G. Potassium and Magnesium in Irrigation Water Quality Assessment. Agric. Water Manag. 2015, 157, 59–64. [Google Scholar] [CrossRef]
- Rácz Istvánné Nemes, Z.J. Öntőzővizek Minősége a Hazai Vízkultúrás Zöldségtermesztésben; Corvinus University of Budapest: Budapest, Hungary, 2007. [Google Scholar]
- Sarda, P.; Sadgir, P. Assessment of Multi Parameters of Water Quality in Surface Water Bodies—A Review. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 331–336. [Google Scholar]
- Rengasamy, P.; Olsson, K.A. Irrigation and Sodicity. Aust. J. Soil Res. 1993, 31, 821–837. [Google Scholar] [CrossRef]
- Halliwell, D.J.; Barlow, K.M.D.M.; Nash, D.M. A Review of the Effects of Wastewater Sodium on Soil Physical Properties and Their Implications for Irrigation Systems. Aust. J. Soil Res. 2001, 39, 1259–1267. [Google Scholar] [CrossRef]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium Replacement of Potassium in Physiological Processes of Olive Trees (Var. Barnea) as Affected by Drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S.; Oster, J.D.; Sposito, G.; Minhas, P.S.; Cheraghi, S.A.M.; Murtaza, G.; Mirzabaev, A.; Saqib, M. High-magnesium Waters and Soils: Emerging Environmental and Food Security Constraints. Sci. Total Environ. 2018, 642, 1108–1117. [Google Scholar] [CrossRef]
- Martínez-Gimeno, M.A.; Bonet, L.; Provenzano, G.; Badal, E.; Intrigliolo, D.S.; Ballester, C. Assessment of Yield and Water Productivity of Clementine Trees under Surface and Subsurface Drip Irrigation. Agric. Water Manag. 2018, 206, 209–216. [Google Scholar] [CrossRef]
- Sable, R.; Kolekar, S.; Gawde, A.; Takle, S.; Pednekar, A. A Review on Different Irrigation Methods. Int. J. Appl. Agric. Res. 2019, 14, 49–60. [Google Scholar]
- Holzapfel, E.A.; Pannunzio, A.; Lorite, I.; de Oliveira, A.S.S.; Farkas, I. Design and Management of Irrigation Systems. Chil. J. Agric. Res. 2009, 69, 17–25. [Google Scholar] [CrossRef]
- Pereira, L.S. Surface Irrigation Systems. Sustain. Irrig. Agric. 1996, 269–289. [Google Scholar] [CrossRef]
- Albaji, M.; Golabi, M.; Boroomand Nasab, S.; Zadeh, F.N. Investigation of Surface, Sprinkler and Drip Irrigation Methods Based on the Parametric Evaluation Approach in Jaizan Plain. J. Saudi Soc. Agric. Sci. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Nakayama, F.S.; Bucks, D.A. Water Quality in Drip/Trickle Irrigation: A Review. Irrig. Sci. 1991, 12, 187–192. [Google Scholar] [CrossRef]
- Yan, H.; Hui, X.; Li, M.; Xu, Y. Development in Sprinkler Irrigation Technology in China. Irrig. Drain. 2020, 69, 75–87. [Google Scholar] [CrossRef]
- Bansal, G.; Mahajan, A.; Verma, A.; Bandhu Singh, D. A Review on Materialistic Approach to Drip Irrigation System. Mater. Today Proc. 2021, 46, 10712–10717. [Google Scholar] [CrossRef]
- Henrique Bassoi, L.; Hopmans, J.W.; André de Castro Jorge, L.; Miranda de Alencar, C.; Antonio Moura Silva, J. Grapevine Root Distribution in drip and microsprinkler irrigation. Sci. Agric. 2003, 60, 377–387. [Google Scholar] [CrossRef]
- Goldberg, D.; Gornat, B.; Rimon, D. Drip Irrigation. Principles, Design and Agricultural Practices; Drip Irrigation Scientific Publications: Kfar Shmaryahu, Israel, 1976. [Google Scholar]
- Dong, R.; Liu, W.; Qu, J.; Cao, W. Accumulation of Na+ in Cotton Field under Mulched Drip Irrigation of Brackish Water in Arid Areas. Separations 2023, 10, 180. [Google Scholar] [CrossRef]
- Zapata, N.; Playán, E.; Castillo, R.; Gimeno, Y.; Oliván, I.; Jiménez, A.; Carbonell, X.; Fábregas, M.; López-Pardo, J.R.; Vicente, L.M.; et al. A Methodology to Classify Irrigated Areas: Application to the Central Ebro River Basin in Aragón (Spain). Agric. Water Manag. 2020, 241, 106365. [Google Scholar] [CrossRef]
- Arshad, I. Importance of Drip Irrigation System Installation and Management—A Review. PSM Biol. Res. 2020, 5, 22–29. [Google Scholar]
- Flores, J.H.N.; Faria, L.C.; Neto, O.R.; Diotto, A.V.; Colombo, A. Methodology for Determining the Emitter Local Head Loss in Drip Irrigation Systems. J. Irrig. Drain. Eng. 2020, 147, 06020014. [Google Scholar] [CrossRef]
- Shi, K.; Lu, T.; Zheng, W.; Zhang, X.; Zhangzhong, L. A Review of the Category, Mechanism, and Controlling Methods of Chemical Clogging in Drip Irrigation System. Agriculture 2022, 12, 1–20. [Google Scholar] [CrossRef]
- Prathyusha, K.; Chaitanya Suman, M. Design of Embedded Systems for the Automation of Drip Irrigation. Int. J. Appl. Innov. Eng. Manag. 2012, 1, 245–258. [Google Scholar]
- Li, Y.; Pan, J.; Chen, X.; Xue, S.; Feng, J.; Muhammad, T.; Zhou, B. Dynamic Effects of Chemical Precipitates on Drip Irrigation System Clogging Using Water with High Sediment and Salt Loads. Agric. Water Manag. 2019, 213, 833–842. [Google Scholar] [CrossRef]
- Bucks, D.A.; Nakayama, F.S.; Gilbert, R.G. Trickle Irrigation Water Quality and Preventive Maintenance. Agric. Water Manag. 1979, 2, 149–162. [Google Scholar] [CrossRef]
- Pitts, D.J.; Haman, D.Z.; Smajstria, A.G. Causes and Prevention of Emitter Plugging in Micro Irrigation Systems; Bulletin 2; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1990. [Google Scholar]
- Muhammad, T.; Zhou, B.; Liu, Z.; Chen, X.; Li, Y. Effects of Phosphorus-Fertigation on Emitter Clogging in Drip Irrigation System with Saline Water. Agric. Water Manag. 2021, 243, 106392. [Google Scholar] [CrossRef]
- El Housse, M.; Hadfi, A.; Karmal, I.; Ben-Aazza, S.; Belattar, M.; Errami, M.; Mohareb, S.; Driouiche, A. Study of the Effect of Inorganic Inhibitor on the Calcium Carbonate Precipitation in the Localized Irrigation Systems. Nanotechnol. Environ. Eng. 2021, 6, 2–9. [Google Scholar] [CrossRef]
- Yan, D.; Bai, Z.; Rowan, M.; Gu, L.; Shumei, R.; Yang, P. Biofilm Structure and Its Influence on Clogging in Drip Irrigation Emitters Distributing Reclaimed Wastewater. J. Environ. Sci. 2009, 21, 834–841. [Google Scholar] [CrossRef]
- Ravina, I.; Paz, E.; Sofer, Z.; Marcu, A.; Schischa, A.; Sagi, G.; Yechialy, Z.; Lev, Y. Control of Clogging in Drip Irrigation with Stored Treated Municipal Sewage Effluent. Agric. Water Manag. 1997, 33, 127–137. [Google Scholar] [CrossRef]
- Dery, J.L.; Brassill, N.; Rock, C.M. Minimizing Risks: Use of Surface Water in Pre-Harvest Agricultural Irrigation Part I: Understanding Water Quality & Treatment Options Why Treat Agricultural Irrigation Water? Univ. Arizona Coop. Ext. 2019, 1793, 1–4. [Google Scholar]
- Lim, K.Y.; Foo, K.Y. Hazard Identification and Risk Assessment of the Organic, Inorganic and Microbial Contaminants in the Surface Water after the High Magnitude of Flood Event. Environ. Int. 2021, 157, 106851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ao, C.; Bailey, R.T.; Zeng, W.; Huang, J. Simulation of Water and Salt Transport in the Kaidu River Irrigation District Using the Modified SWAT-Salt. Agric. Water Manag. 2022, 272, 107845. [Google Scholar] [CrossRef]
- Battilani, A.; Steiner, M.; Andersen, M.; Back, S.N.; Lorenzen, J.; Schweitzer, A.; Dalsgaard, A.; Forslund, A.; Gola, S.; Klopmann, W.; et al. Decentralised Water and Wastewater Treatment Technologies to Produce Functional Water for Irrigation. Agric. Water Manag. 2010, 98, 385–402. [Google Scholar] [CrossRef]
- Vassalle, L.; Sunyer-Caldú, A.; Uggetti, E.; Díez-Montero, R.; Díaz-Cruz, M.S.; García, J.; García-Galán, M.J. Bioremediation of Emerging Micropollutants in Irrigation Water. The Alternative of Microalgae-Based Treatments. J. Environ. Manag. 2020, 274. [Google Scholar] [CrossRef] [PubMed]
- Gertsis, A.; Zoukidis, K. Irrigation with Highly Saline Water: A New Innovative Water Treatment System Evaluated for Vegetable Production in Greenhouse. Eur. Water 2017, 59, 331–337. [Google Scholar]
- Seyyedi, M. Magnetic Fields and Manipulation of Water Behaviors: A Viable Method for Reducing RO Specific Energy Consumption. In Proceedings of the 2022 Membrane Technology Conference and Exposition, Las Vegas, NV, USA, 21–25 February 2022. [Google Scholar]
- Surendran, U.; Sandeep, O.; Joseph, E.J. The Impacts of Magnetic Treatment of Irrigation Water on Plant, Water and Soil Characteristics. Agric. Water Manag. 2016, 178, 21–29. [Google Scholar] [CrossRef]
- Hachicha, M.; Kahlaoui, B.; Khamassi, N.; Misle, E.; Jouzdan, O. Effect of Electromagnetic Treatment of Saline Water on Soil and Crops. J. Saudi Soc. Agric. Sci. 2018, 17, 154–162. [Google Scholar] [CrossRef]
- Wiedenfeld, B. Effects of Irrigation Water Salinity and Electrostatic Water Treatment for Sugarcane Production. Agric. Water Manag. 2008, 95, 85–88. [Google Scholar] [CrossRef]
- Wei, K.; Zhang, J.; Wang, Q.; Guo, Y.; Mu, W. Irrigation with Ionized Brackish Water Affects Cotton Yield and Water Use Efficiency. Ind. Crops Prod. 2022, 175, 114244. [Google Scholar] [CrossRef]
- Jarma, Y.A.; Karaoğlu, A.; Senan, I.R.A.; Meriç, M.K.; Kukul, Y.S.; Özçakal, E.; Barlas, N.T.; Çakıcı, H.; Baba, A.; Kabay, N. Utilization of Membrane Separation Processes for Reclamation and Reuse of Geothermal Water in Agricultural Irrigation of Tomato Plants-Pilot Membrane Tests and Economic Analysis. Desalination 2022, 528, 115608. [Google Scholar] [CrossRef]
- Garg, N.; Choudhary, O.P.; Thaman, S.; Sharma, V.; Singh, H.; Vashistha, M.; Sekhon, K.S.; Sharda, R.; Dhaliwal, M.S. Effects of Irrigation Water Quality and NPK-Fertigation Levels on Plant Growth, Yield and Tuber Size of Potatoes in a Sandy Loam Alluvial Soil of Semi-Arid Region of Indian Punjab. Agric. Water Manag. 2022, 266, 107604. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Zhang, J.; Ma, K. An Optimum Combination of Irrigation Amount, Irrigation Water Salinity and Nitrogen Application Rate Can Improve Cotton (for Fiber) Nitrogen Uptake and Final Yield. Ind. Crops Prod. 2022, 187, 115386. [Google Scholar] [CrossRef]
- Sang, H.; Guo, W.; Gao, Y.; Jiao, X.; Pan, X. Effects of Alternating Fresh and Saline Water Irrigation on Soil Salinity and Chlorophyll Fluorescence of Summer Maize. Water 2020, 12, 3054. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Zhang, X.; Dang, H.; Singh, B.P.; Liu, X.; Sun, H. Long-Term Saline Water Irrigation Decreased Soil Organic Carbon and Inorganic Carbon Contents. Agric. Water Manag. 2022, 270, 107760. [Google Scholar] [CrossRef]
- Ouoba, N.; Phung, L.D.; Sasaki, A.; Pham, D.V.; Watanabe, T. Drip Fertigation with Treated Municipal Wastewater and Soil Amendment with Composted Sewage Sludge for Sustainable Protein-Rich Rice Cultivation. Environ. Technol. Innov. 2022, 28, 102569. [Google Scholar] [CrossRef]
- Yang, S.; Feng, W.; Wang, S.; Chen, L.; Zheng, X.; Li, X.; Zhou, D. Farmland Heavy Metals Can Migrate to Deep Soil at a Regional Scale: A Case Study on a Wastewater-Irrigated Area in China. Environ. Pollut. 2021, 281, 116977. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, X.; Wang, Y.; Shi, E.; Lin, H.; Chen, G. Removal of Pb2+ and Cd2+ from Irrigation Water and Replenishment of Mineral Nutrients Using a Low-Cost Mineral Adsorbent Derived from Potassium-Rich Aluminum Silicates. J. Environ. Chem. Eng. 2023, 11, 109282. [Google Scholar] [CrossRef]
- Amiri, M.J.; Bahrami, M.; Badkouby, M.; Kalavrouziotis, I.K. Greywater Treatment Using Single and Combined Adsorbents for Landscape Irrigation. Environ. Process. 2019, 6, 43–63. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption Isotherm, Kinetic Modeling and Mechanism of 2,4,6-Trichlorophenol on Coconut Husk-Based Activated Carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of Plant Based Biosorbents in the Removal of Heavy Metals: A Review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Aghakhani, A.; Mousavi, S.F.; Mostafazadeh-Fard, B.; Rostamian, R.; Seraji, M. Application of Some Combined Adsorbents to Remove Salinity Parameters from Drainage Water. Desalination 2011, 275, 217–223. [Google Scholar] [CrossRef]
- Ghanbarizadeh, P.; Parivazh, M.M.; Abbasi, M.; Osfouri, S.; Dianat, M.J.; Rostami, A.; Dibaj, M.; Akrami, M. Performance Enhancement of Specific Adsorbents for Hardness Reduction of Drinking Water and Groundwater. Water 2022, 14, 2749. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of Heavy Metal Ions from Aqueous Solution by Zeolite Synthesized from Fly Ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Paul, B.; Dynes, J.J.; Chang, W. Modified Zeolite Adsorbents for the Remediation of Potash Brine-Impacted Groundwater: Built-in Dual Functions for Desalination and PH Neutralization. Desalination 2017, 419, 141–151. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Mohamed, A.M.G.; Keshawy, M.; elMoghny, T.A.; Shehata, N. Adsorption of Heavy Metals and Hardness Ions from Groundwater onto Modified Zeolite: Batch and Column Studies. Alex. Eng. J. 2022, 61, 4189–4207. [Google Scholar] [CrossRef]
- Raheem, S.A.; Kadhim, E.J.; Abdulhasan, M.J. Comparative Study of Iron Removal from Groundwater Using Low Cost Adsorbents. J. Ecol. Eng. 2022, 23, 18–23. [Google Scholar] [CrossRef]
- Kan, C.C.; Aganon, M.C.; Futalan, C.M.; Dalida, M.L.P. Adsorption of Mn2+ from Aqueous Solution Using Fe and Mn Oxide-Coated Sand. J. Environ. Sci. 2013, 25, 1483–1491. [Google Scholar] [CrossRef]
- Fadel, M.; Hassanein, N.M.; Elshafei, M.M.; Mostafa, A.H.; Ahmed, M.A.; Khater, H.M. Biosorption of Manganese from Groundwater by Biomass of Saccharomyces Cerevisiae. HBRC J. 2017, 13, 106–113. [Google Scholar] [CrossRef]
- Kamarudzaman, A.N.; Chay, T.C.; Amir, A.; Talib, S.A. Biosorption of Mn(II) Ions from Aqueous Solution by Pleurotus Spent Mushroom Compost in a Fixed-Bed Column. Procedia—Soc. Behav. Sci. 2015, 195, 2709–2716. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K. Conventional and Non-Conventional Adsorbents for Removal of Pollutants from Water—A Review. Indian J. Chem. Technol. 2006, 13, 203–217. [Google Scholar]
- McKay, G.; Bino, M.J.; Altamemi, A.R. The Adsorption of Various Pollutants from Aqueous Solutions on to Activated Carbon. Water Res. 1985, 19, 491–495. [Google Scholar] [CrossRef]
- Leboda, R. Carbon-Mineral Adsorbents—New Type of Sorbents Part II. Surface Properties and Methods of Their Modification. Mater. Chem. Phys. 1993, 34, 123–141. [Google Scholar] [CrossRef]
- Gabaldón, C.; Marzal, P.; Seco, A.; Gonzalez, J.A. Cadmium and Copper Removal by a Granular Activated Carbon in Laboratory Column Systems. Sep. Sci. Technol. 2000, 35, 1039–1053. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A Review of Emerging Adsorbents for Nitrate Removal from Water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Aghakhani, A.; Mousavi, S.F.; Mostafazadeh-Fard, B. Desalination of Saline Water with Single and Combined Adsorbents. Desalin. Water Treat. 2013, 51, 1928–1935. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Alsultan, M.; Sabah, A.A.; Swiegers, G.F. Carbon Dioxide Adsorption by a High-Surface-Area Activated Charcoal. J. Compos. Sci. 2023, 7, 179. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Carrott, M.R. Lignin—From Natural Adsorbent to Activated Carbon: A Review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Ahmed, M.; Chin, Y.H.; Guo, X.; Zhao, X.M. Microwave Assisted Digestion Followed by ICP-MS for Determination of Trace Metals in Atmospheric and Lake Ecosystem. J. Environ. Sci. 2017, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mattson, J.S.; Mark, H.B. Activated Carbon: Surface Chemistry and Adsorption from Solution; M. Dekker: New York, NY, USA, 1971; ISBN 0824714431. [Google Scholar]
- Chiang, Y.C.; Chiang, P.C.; Chiang, H.L.; Chang, E.E. Determination of Chemical Characterizations of Activated Carbons from Various Raw Materials. Toxicol. Environ. Chem. 1998, 67, 275–291. [Google Scholar] [CrossRef]
- Grant, G.A.; Fisher, P.R.; Barrett, J.E.; Wilson, P.C. Removal of Agrichemicals from Water Using Granular Activated Carbon Filtration. Water. Air. Soil Pollut. 2019, 230, 7. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The Role of Nanomaterials as Effective Adsorbents and Their Applications in Wastewater Treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Adsorption of Heavy Metal Ions with Carbon Nanotubes. Sep. Purif. Technol. 2007, 58, 49–52. [Google Scholar] [CrossRef]
- Mumpton, F.A. La Roca Magica: Uses of Natural Zeolites in Agriculture and Industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Dong, H.; Zeng, G. Application of Zeolite in Removing Salinity/Sodicity from Wastewater: A Review of Mechanisms, Challenges and Opportunities. J. Clean. Prod. 2018, 197, 1435–1446. [Google Scholar] [CrossRef]
- El Bastamy, E.; Ibrahim, L.A.; Ghandour, A.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes. Sustainability 2021, 13, 5738. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Gibb, N.P.; Dynes, J.J.; Chang, W. Synergistic Desalination of Potash Brine-Impacted Groundwater Using a Dual Adsorbent. Sci. Total Environ. 2017, 593–594, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gibb, N.P.; Dynes, J.J.; Chang, W. A Recyclable Adsorbent for Salinized Groundwater: Dual-Adsorbent Desalination and Potassium-Exchanged Zeolite Production. Chemosphere 2018, 209, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.P.; Aris, A.Z. A Review on Economically Adsorbents on Heavy Metals Removal in Water and Wastewater. Rev. Environ. Sci. Biotechnol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural Waste Peels as Versatile Biomass for Water Purification—A Review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Younas, H.; Younas, F. Wastewater Application in Agriculture—A Review. Water Air Soil Pollut. 2022, 233, 329. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The Improved Methods of Heavy Metals Removal by Biosorbents: A Review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Jesus, J.; Nunes da Silva, R.; Pintor, A. Advances in Cork Use in Adsorption Applications: An Overview of the Last Decade of Research. Separations 2023, 10, 1–18. [Google Scholar] [CrossRef]
- Yan, S.; Yu, W.; Yang, T.; Li, Q.; Guo, J. The Adsorption of Corn Stalk Biochar for Pb and Cd: Preparation, Characterization, and Batch Adsorption Study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, I.; Usman, M.; Iqbal, R.; Rizwan, M.; Abdel-Hameed, U.K.; Haider, A.A.; Tariq, A. Biochar as a Green Sorbent for Remediation of Polluted Soils and Associated Toxicity Risks: A Critical Review. Separations 2023, 10, 197. [Google Scholar] [CrossRef]


| Positive Effects | Negative Effects |
|---|---|
| better plant water supply | structural damage |
| increased nutrient access | deterioration of topsoil water management |
| increased nutrient intake | possible leaching of nutrients |
| leaching of harmful salts | salinization |
| protection against erosion and deflation | in case of overwatering reduction, rise in groundwater level, waterlogging |
| Type of Adsorbent | Type of Pollutants | pH | Adsorption Efficiency (mgg−1) | References |
|---|---|---|---|---|
| Conventional adsorbents | ||||
| Activated carbon + cation resin | K+ | 4.50 | 0.26 | [88] |
| Na+ | 4.50 | 13.80 | ||
| Ca2+ | 4.50 | 2.50 | ||
| Mg2+ | 4.50 | 2.73 | ||
| Cl− | 4.50 | 11.50 | ||
| HCO3− | 4.50 | 18.30 | ||
| Activated carbon + zeolite | K+ | 7.20 | 0.05 | [88] |
| Na+ | 7.20 | 2.10 | ||
| Ca2+ | 7.20 | 1.00 | ||
| Mg2+ | 7.20 | 0.12 | ||
| Cl− | 7.20 | 0.90 | ||
| HCO3− | 7.20 | 4.00 | ||
| Activated carbon | Hardness | 0.86 | [89] | |
| Zeolite | Mn2+ | 8.70 | 30.89 | [90] |
| Thermally activated natural zeolite (NZ 200) | Fe2+ | 8.95 | 6.13 | [91] |
| Mn2+ | 8.95 | 0.86 | ||
| Modified zeolite | Na+ | 7.87 ± 0.25 | N/A | [92] |
| TiO2@Zeolite | Mn2+ | 7.00 | 94.10 | [93] |
| Fe3+ | 7.00 | 150.10 | ||
| Hardness | 7.00 | 131.8 (Ca2+) 703.6 (T.H.) * | ||
| Activated carbon + nano zero-valent iron + natural zeolite | COD | 6.50–8.50 | 35.64 | [83] |
| Zeolite clinoptilolite | Hardness | 7.48 | [89] | |
| Activated alumina | Hardness | 44954.00 | [89] | |
| Non-conventional adsorbents | ||||
| Sawdust and barley husk | Fe2+ | 6.50 | N/A | [94] |
| Manganese oxide-coated sand1 (MOCS1) | Mn2+ | 8.00 | 2.61 | [95] |
| Manganese oxide-coated sand2 (MOCS2) | 0.83 | |||
| Iron oxide-coated sand1 (IOCS2) | 0.40 | |||
| Iron oxide-coated sand2 (IOCS2) | 0.55 | |||
| Manganese and iron oxide-coated sand (MIOCS) | 0.88 | |||
| Saccharomyces cerevisiae | Mn2+ | 4.50–9.00 | N/A | [96] |
| Pleurotus mushroom compost | Mn2+ | 6.00 | N/A | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucserka, T.; Németh, G.I.; Pálfi, I.; Kiss, Z.L.; Tombácz, E.; Galambos, I. Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues. Separations 2023, 10, 468. https://doi.org/10.3390/separations10090468
Kucserka T, Németh GI, Pálfi I, Kiss ZL, Tombácz E, Galambos I. Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues. Separations. 2023; 10(9):468. https://doi.org/10.3390/separations10090468
Chicago/Turabian StyleKucserka, Tamás, Gábor István Németh, Ivett Pálfi, Zsolt L. Kiss, Etelka Tombácz, and Ildikó Galambos. 2023. "Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues" Separations 10, no. 9: 468. https://doi.org/10.3390/separations10090468
APA StyleKucserka, T., Németh, G. I., Pálfi, I., Kiss, Z. L., Tombácz, E., & Galambos, I. (2023). Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues. Separations, 10(9), 468. https://doi.org/10.3390/separations10090468