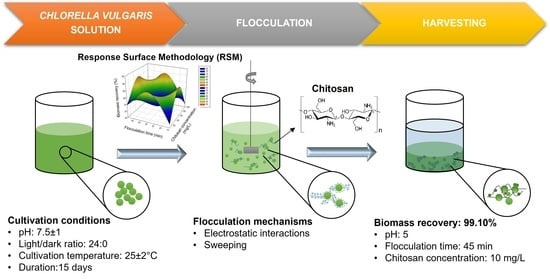
Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Culture Conditions
2.2. Experimental Procedure and Analytical Methods
Mixing Speed
2.3. Experimental Design and Data Analysis
2.4. A Brief Feasibility Assessment and Cost Estimate of Chitosan
3. Results and Discussion
3.1. Evaluation of Operating Parameters
3.2. Evaluation of RSM Models
3.3. Statistical Analysis of Factors
3.4. Analysis of Variables
3.5. Feasibility Assessment and Cost Analysis of Chitosan
| Flocculant | Biomass (mg/L) | Flocculant Dose (mg/L) | Flocculant Efficiency (%) | Required Flocculant Dose (ton ton−1 biomass) | Flocculant Cost (US$ ton−1) | Required Flocculant Cost (US$ ton−1 biomass) | Ref. |
|---|---|---|---|---|---|---|---|
| Al2(SO4)3 | 250 | 20 | 85 | 0.094 | 300 | 28 | [67] |
| Ca(OH)2 | 500 | n.a. | n.a. | 0.120 | 150 | 18 | [68] |
| NaOH | 500 | n.a. | n.a. | 0.120 | 350 | 42 | [69] |
| Flopam | 260 | 5 | 98 | 0.020 | 8000 | 157 | [58] |
| Zetag | 260 | 5 | 100 | 0.019 | 8000 | 154 | [58] |
| Chitosan | 590 | 5 | 98.9 | 0.0084 | 20,984 | 176.81 | [30] |
| Chitosan | 373 ± 87 | 10 | 99 | 0.021–0.035 | 1000 | 21–35 | This study |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, J.R.d.S.; Santos, R.d.S.; Matos, R.A.; Pires, J.C.; Salgado, E.M. Sustainable Microalgal Harvesting Process Applying Opuntia cochenillifera: Process Parameters Optimization. Appl. Sci. 2023, 13, 1203. [Google Scholar] [CrossRef]
- Singh, B.; Guldhe, A.; Rawat, I.; Bux, F. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew. Sustain. Energy Rev. 2014, 29, 216–245. [Google Scholar]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Integrating first, second, and third generation biorefineries: Incorporating microalgae into the sugarcane biorefinery. Chem. Eng. Sci. 2014, 118, 126–140. [Google Scholar]
- Lananan, F.; Hamid, S.H.A.; Din, W.N.S.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Prandini, J.M.; Da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Zhu, L. Self-flocculation as an efficient method to harvest microalgae: A mini-review. Water 2021, 13, 2585. [Google Scholar] [CrossRef]
- Nitsos, C.; Filali, R.; Taidi, B.; Lemaire, J. Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv. 2020, 45, 107650. [Google Scholar]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Estime, B.; Ren, D.; Sureshkumar, R. Cultivation and energy efficient harvesting of microalgae using thermoreversible sol-gel transition. Sci. Rep. 2017, 7, 40725. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.-H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Seo, Y.H.; Park, D.; Oh, Y.-K.; Yoon, S.; Han, J.-I. Harvesting of microalgae cell using oxidized dye wastewater. Bioresour. Technol. 2015, 192, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.S.; Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 102046. [Google Scholar]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar]
- Ferreira, J.; de Assis, L.R.; de Sousa Oliveira, A.P.; de Siqueira Castro, J.; Calijuri, M.L. Innovative microalgae biomass harvesting methods: Technical feasibility and life cycle analysis. Sci. Total Environ. 2020, 746, 140939. [Google Scholar] [PubMed]
- Elcik, H.; Cakmakci, M. Harvesting of microalgae via submerged membranes: Flux, fouling and its reversibility. Membr. Water Treat. 2017, 8, 499–515. [Google Scholar]
- Shaikh, S.M.; Hassan, M.K.; Nasser, M.S.; Sayadi, S.; Ayesh, A.I.; Vasagar, V. A comprehensive review on harvesting of microalgae using Polyacrylamide-Based Flocculants: Potentials and challenges. Sep. Purif. Technol. 2021, 277, 119508. [Google Scholar]
- Ananthi, V.; Balaji, P.; Sindhu, R.; Kim, S.-H.; Pugazhendhi, A.; Arun, A. A critical review on different harvesting techniques for algal based biodiesel production. Sci. Total Environ. 2021, 780, 146467. [Google Scholar]
- Goswami, G.; Kumar, R.; Sinha, A.; Maiti, S.K.; Dutta, B.C.; Singh, H.; Das, D. A low-cost and scalable process for harvesting microalgae using commercial-grade flocculant. RSC Adv. 2019, 9, 39011–39024. [Google Scholar]
- Roselet, F.; Vandamme, D.; Muylaert, K.; Abreu, P.C. Harvesting of microalgae for biomass production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 211–243. [Google Scholar]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M.P. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2021, 137, 110498. [Google Scholar]
- Rashid, N.; Rehman, S.U.; Han, J.-I. Rapid harvesting of freshwater microalgae using chitosan. Process Biochem. 2013, 48, 1107–1110. [Google Scholar]
- Mubarak, M.; Shaija, A.; Suchithra, T. Flocculation: An effective way to harvest microalgae for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103221. [Google Scholar]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of microalgae by flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Vermuë, M.; Olivieri, G.; van den Broek, L.; Barbosa, M.; Eppink, M.; Wijffels, R.; Kleinegris, D. Cationic polymers for successful flocculation of marine microalgae. Bioresour. Technol. 2014, 169, 804–807. [Google Scholar]
- Niemi, C.; Gentili, F.G. The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol. Plant. 2021, 173, 536–542. [Google Scholar] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [PubMed]
- Ahmad, A.; Yasin, N.M.; Derek, C.; Lim, J. Optimization of microalgae coagulation process using chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar]
- Yin, Z.; Hu, D.; Li, X.; Zhong, Y.; Zhu, L. Shell-derived chitosan as a green flocculant to harvest microalgae for biofuel production. Biofuels Bioprod. Biorefin. 2021, 15, 637–645. [Google Scholar]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Chen, S.; Chung, Y.-C. Evaluation of a modified chitosan biopolymer for coagulation of colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1999, 147, 359–364. [Google Scholar]
- Adesina, O.A.; Abdulkareem, F.; Yusuff, A.S.; Lala, M.; Okewale, A. Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. S. Afr. J. Chem. Eng. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Das, D.; Vimala, R.; Das, N. Biosorption of Zn (II) onto Pleurotus platypus: 5-Level Box–Behnken design, equilibrium, kinetic and regeneration studies. Ecol. Eng. 2014, 64, 136–141. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Xu, P.-C.; Huang, G.-H.; Chai, T.; Hou, M.; Gao, P.-F. Effects of aeration parameters on effluent quality and membrane fouling in a submerged membrane bioreactor using Box–Behnken response surface methodology. Desalination 2012, 302, 33–42. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Lin, X.; Xu, Z.; Luo, W.; Luo, L. Response surface methodology to optimize self-flocculation harvesting of microalgae Desmodesmus sp. CHX1. Environ. Technol. 2022, 43, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.M.; Tyagi, V.; Ahmad, S.; Kothari, R. Optimization of flocculation efficiency of Chlorella pyrenoidosa with CaCl2 using the Box-Behnken design of response surface methodology: A cost effective statistical investigation. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M. Evaluation of flocculation performance of amphoteric flocculant when harvesting microalgae Coccomyxa sp. KJ by response surface methodology. J. Environ. Manag. 2021, 277, 111449. [Google Scholar] [CrossRef]
- Pérez, L.; Salgueiro, J.L.; Maceiras, R.; Cancela, Á.; Sánchez, Á. Study of influence of pH and salinity on combined flocculation of Chaetoceros gracilis microalgae. Chem. Eng. J. 2016, 286, 106–113. [Google Scholar] [CrossRef]
- Silva, D.A.; Cardoso, L.G.; de Jesus Silva, J.S.; de Souza, C.O.; Lemos, P.V.F.; de Almeida, P.F.; de Souza Ferreira, E.; Lombardi, A.T.; Druzian, J.I. Strategy for the cultivation of Chlorella vulgaris with high biomass production and biofuel potential in wastewater from the oil industry. Environ. Technol. Innov. 2022, 25, 102204. [Google Scholar] [CrossRef]
- Elcik, H.; Cakmakci, M.; Ozkaya, B. Preparation and characterisation of novel polysulfone membranes modified with Pluronic F-127 for reducing microalgal fouling. Chem. Pap. 2017, 71, 1271–1290. [Google Scholar] [CrossRef]
- Elcik, H.; Cakmakci, M.; Ozkaya, B. The fouling effects of microalgal cells on crossflow membrane filtration. J. Membr. Sci. 2016, 499, 116–125. [Google Scholar] [CrossRef]
- Karnena, M.K.; Dwarapureddi, B.K.; Saritha, V. Alum, Chitin and Sago as coagulants for the optimization of process parameters focussing on coagulant dose and mixing speed. Watershed Ecol. Environ. 2022, 4, 112–124. [Google Scholar] [CrossRef]
- Ma, W.; Feng, C.; Guan, F.; Ma, D.; Cai, J. Effective Chlorella vulgaris Biomass Harvesting through Sulfate and Chloride Flocculants. J. Mar. Sci. Eng. 2023, 11, 47. [Google Scholar] [CrossRef]
- Loganathan, K.; Saththasivam, J.; Sarp, S. Removal of microalgae from seawater using chitosan-alum/ferric chloride dual coagulations. Desalination 2018, 433, 25–32. [Google Scholar] [CrossRef]
- Barekati-Goudarzi, M.; Reza Mehrnia, M.; Pourasgharian Roudsari, F.; Boldor, D. Rapid separation of microalga Chlorella vulgaris using magnetic chitosan: Process optimization using response surface methodology. Part. Sci. Technol. 2016, 34, 165–172. [Google Scholar]
- Tran, D.-T.; Le, B.-H.; Lee, D.-J.; Chen, C.-L.; Wang, H.-Y.; Chang, J.-S. Microalgae harvesting and subsequent biodiesel conversion. Bioresour. Technol. 2013, 140, 179–186. [Google Scholar]
- Demir, I.; Blockx, J.; Dague, E.; Guiraud, P.; Thielemans, W.; Muylaert, K.; Formosa-Dague, C. Nanoscale evidence unravels microalgae flocculation mechanism induced by chitosan. ACS Appl. Bio Mater. 2020, 3, 8446–8459. [Google Scholar] [CrossRef]
- Demir, I.; Besson, A.; Guiraud, P.; Formosa-Dague, C. Towards a better understanding of microalgae natural flocculation mechanisms to enhance flotation harvesting efficiency. Water Sci. Technol. 2020, 82, 1009–1024. [Google Scholar] [CrossRef]
- Blockx, J.; Verfaillie, A.; Thielemans, W.; Muylaert, K. Unravelling the mechanism of chitosan-driven flocculation of microalgae in seawater as a function of pH. ACS Sustain. Chem. Eng. 2018, 6, 11273–11279. [Google Scholar] [CrossRef]
- Miranda, R.; Nicu, R.; Latour, I.; Lupei, M.; Bobu, E.; Blanco, A. Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation. Chem. Eng. J. 2013, 231, 304–313. [Google Scholar]
- Joglekar, A.; May, A. Product excellence through design of experiments. Cereal Foods World 1987, 32, 857. [Google Scholar]
- Banerjee, A.; Sarkar, P.; Banerjee, S. Application of statistical design of experiments for optimization of As (V) biosorption by immobilized bacterial biomass. Ecol. Eng. 2016, 86, 13–23. [Google Scholar]
- Körbahti, B.K. Response surface optimization of electrochemical treatment of textile dye wastewater. J. Hazard. Mater. 2007, 145, 277–286. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Ghosh, S.; Sen, G.; Mishra, S.; Shukla, P.; Bandopadhyay, R. Study of algal biomass harvesting through cationic cassia gum, a natural plant based biopolymer. Bioresour. Technol. 2014, 151, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar]
- El-Qanni, A.; Alsayed, M.; Alsurakji, I.H.; Najjar, M.; Odeh, D.; Najjar, S.; Hmoudah, M.; Zubair, M.; Russo, V.; Di Serio, M. A technoeconomic assessment of biological sludge dewatering using a thermal rotary dryer: A case study of design applicability, economics, and managerial feasibility. Biomass Convers. Biorefin. 2022, 1–15. [Google Scholar] [CrossRef]
- Roselet, F.; Vandamme, D.; Roselet, M.; Muylaert, K.; Abreu, P.C. Screening of commercial natural and synthetic cationic polymers for flocculation of freshwater and marine microalgae and effects of molecular weight and charge density. Algal Res. 2015, 10, 183–188. [Google Scholar]
- Beach, E.S.; Eckelman, M.J.; Cui, Z.; Brentner, L.; Zimmerman, J.B. Preferential technological and life cycle environmental performance of chitosan flocculation for harvesting of the green algae Neochloris oleoabundans. Bioresour. Technol. 2012, 121, 445–449. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, M.; Guldhe, A.; Ansari, F.; Rawat, I.; Kanney, K.; Bux, F. Design and development of polyamine polymer for harvesting microalgae for biofuels production. Energy Convers. Manag. 2014, 85, 537–544. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.-C.; Han, J.-I.; Darpito, C.H.; Choi, M.; Yang, J.-W. Efficient microalgae harvesting by organo-building blocks of nanoclays. Green Chem. 2013, 15, 749–755. [Google Scholar] [CrossRef]
- Lee, S.-M.; Choi, H.-J. Harvesting of microalgae species using Mg–sericite flocculant. Bioprocess Biosyst. Eng. 2015, 38, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of microalgae by flocculation with poly (γ-glutamic acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Granados, M.; Acién, F.; Gómez, C.; Fernández-Sevilla, J.; Grima, E.M. Evaluation of flocculants for the recovery of freshwater microalgae. Bioresour. Technol. 2012, 118, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zou, X.; Mouradov, A.; Spangenberg, G.; Chang, W.; Li, Y. Efficient bioflocculation of Chlorella vulgaris with a chitosan and walnut protein extract. Biology 2021, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.S.; Shariati, A.; Badakhshan, A.; Anvaripour, B. Using nano-chitosan for harvesting microalga Nannochloropsis sp. Bioresour. Technol. 2013, 131, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Muylaert, K. Influence of organic matter generated by Chlorella vulgaris on five different modes of flocculation. Bioresour. Technol. 2012, 124, 508–511. [Google Scholar] [CrossRef]
- Vandamme, D.; Beuckels, A.; Markou, G.; Foubert, I.; Muylaert, K. Reversible flocculation of microalgae using magnesium hydroxide. BioEnergy Res. 2015, 8, 716–725. [Google Scholar] [CrossRef]


| Explanatory Variables | Unit | Symbol | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Chitosan concentration | mg/L | X1 | 10 | 55 | 100 |
| Flocculation time | minute | X2 | 15 | 30 | 45 |
| pH | - | X3 | 5 | 8 | 11 |
| Run | Chitosan Concentration (mg/L) | Flocculation Time (min) | pH | Biomass Recovery (%) | |
|---|---|---|---|---|---|
| Observed Values | Predicted Values | ||||
| 1 | 55 | 30 | 8 | 70.89 | 70.46 |
| 2 | 55 | 15 | 11 | 80.59 | 78.74 |
| 3 | 55 | 30 | 8 | 74.21 | 70.46 |
| 4 | 55 | 30 | 8 | 70.67 | 70.46 |
| 5 | 100 | 30 | 5 | 95.13 | 93.04 |
| 6 | 10 | 30 | 5 | 97.10 | 97.04 |
| 7 | 10 | 30 | 11 | 76.70 | 78.79 |
| 8 | 100 | 45 | 8 | 71.21 | 71.44 |
| 9 | 100 | 15 | 8 | 67.58 | 70.46 |
| 10 | 55 | 45 | 5 | 94.65 | 96.50 |
| 11 | 55 | 15 | 5 | 93.92 | 94.21 |
| 12 | 55 | 30 | 8 | 68.96 | 70.46 |
| 13 | 55 | 45 | 11 | 84.21 | 83.92 |
| 14 | 10 | 45 | 8 | 74.69 | 72.89 |
| 15 | 55 | 30 | 8 | 67.58 | 69.38 |
| 16 | 100 | 30 | 11 | 83.18 | 83.24 |
| 17 | 10 | 15 | 8 | 67.71 | 67.48 |
| Source | Sum of Squares | df | Mean Square | F | p > F |
|---|---|---|---|---|---|
| Sequential model sum of squares | |||||
| Mean | 1.055 × 105 | 1 | 1.055 × 105 | ||
| Linear | 421.76 | 3 | 140.59 | 1.29 | 0.3178 |
| 2FI | 22.74 | 3 | 7.58 | 0.055 | 0.9822 |
| Quadratic | 1341.58 | 3 | 447.19 | 66.35 | <0.0001 |
| Cubic | 22.34 | 3 | 7.45 | 1.20 | 0.4168 |
| Residual | 24.84 | 4 | 6.21 | - | - |
| Total | 1.073 × 105 | 17 | 6311.53 | - | - |
| Lack of fit tests | |||||
| Linear | 1386.67 | 9 | 154.07 | 24.81 | 0.0037 |
| 2FI | 1363.93 | 6 | 227.32 | 36.61 | 0.0019 |
| Quadratic | 22.34 | 3 | 7.45 | 1.20 | 0.4168 |
| Cubic | 0.000 | 0 | - | - | - |
| Pure error | 24.84 | 4 | 6.21 | - | - |
| Source | Model summary statistics | ||||
| Std. dev. | R2 | Adjusted R2 | Predicted R2 | Press | |
| Linear | 10.42 | 0.2301 | 0.0524 | −0.4174 | 2598.40 |
| 2FI | 11.78 | 0.2425 | −0.2121 | −2.0842 | 5654.22 |
| Quadratic | 2.60 | 0.9743 | 0.9412 | 0.7838 | 396.28 |
| Cubic | 2.49 | 0.9865 | 0.9458 | - | - |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 1786.09 | 9 | 198.45 | 29.45 | <0.0001 * |
| X1 | 0.10 | 1 | 0.10 | 0.015 | 0.9059 |
| X2 | 27.98 | 1 | 27.98 | 4.15 | 0.0810 |
| X3 | 393.68 | 1 | 393.68 | 58.41 | 0.0001 * |
| X1X2 | 2.81 | 1 | 2.81 | 0.42 | 0.5394 |
| X1X3 | 17.85 | 1 | 17.85 | 2.65 | 0.1477 |
| X2X3 | 2.09 | 1 | 2.09 | 0.31 | 0.5951 |
| X12 | 0.24 | 1 | 0.24 | 0.036 | 0.8551 |
| X22 | 0.024 | 1 | 0.024 | 3.538 × 10−3 | 0.9542 |
| X32 | 1334.85 | 1 | 1334.85 | 198.06 | <0.0001 * |
| Residual | 47.18 | 7 | 6.74 |
| Flocculant | Experimental Set-Up | Efficiency (%) | Ref. |
|---|---|---|---|
| Nano-aminoclays (Mg-APTES) | BC: 1 g/L; FD: 1 g/L; pH: 5.0–12.0 | >90% | [61] |
| Mg-sericite | BC: 2.13 ± 0.21 g/L; FD: 1–30 mg/L; sericite and MgCl2 ratio (S/M ratio): 40; mixing time: 5 min; mixing rate: 100–150 rpm; settling time: 5 min; pH: 9.0–11.0 | 99 ± 0.3 | [62] |
| Magnetic chitosan | BC: 0.8 OD540 nm; FD: 216 mg/L; pH: 9.0–11.0 | 94 | [45] |
| Poly-γ-glutamic acid | BC: 0.57 g/L; FD: 22.03 mg/L; Salinity: 11.56 g/L; PT: 2 h; pH: 7.5 | 91 | [63] |
| Actipol-FB1 | BC: 1 g/L; FD: 3 mg/L; pH: 8 | 94 | [64] |
| Chitosan | BC: 0.59 g/L; FD: 5 mg/L; PT: 50 min | 98.9 | [30] |
| Chitosan | BC: 0.373 ± 0.087 g/L; FD: 10 mg/L; PT: 46 min. (mixing) + 15 min. (settling); pH: 5 | 99.1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elcik, H.; Karadag, D.; Kara, A.I.; Cakmakci, M. Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology. Separations 2023, 10, 507. https://doi.org/10.3390/separations10090507
Elcik H, Karadag D, Kara AI, Cakmakci M. Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology. Separations. 2023; 10(9):507. https://doi.org/10.3390/separations10090507
Chicago/Turabian StyleElcik, Harun, Dogan Karadag, Ayse Irem Kara, and Mehmet Cakmakci. 2023. "Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology" Separations 10, no. 9: 507. https://doi.org/10.3390/separations10090507
APA StyleElcik, H., Karadag, D., Kara, A. I., & Cakmakci, M. (2023). Microalgae Biomass Harvesting Using Chitosan Flocculant: Optimization of Operating Parameters by Response Surface Methodology. Separations, 10(9), 507. https://doi.org/10.3390/separations10090507