Natural Variation of Volatile Compounds in Virgin Olive Oil Analyzed by HS-SPME/GC-MS-FID
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Olive Oil Extraction
2.3. Analysis of Volatile Compounds
2.4. Statistical Analysis
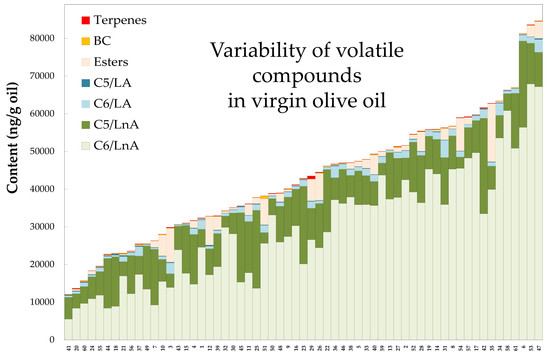
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bernardini, E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Covas, M.I.; Muñoz-Aguayo, D.; Khymenets, O.; de La Torre, R.; Saez, G.; del Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of VOO polyphenols within the frame of the Mediterranean diet: A randomized trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, F.; Ruano, J.; Pérez-Martínez, P.; López-Segura, F.; López-Miranda, J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Olias, J.M.; Perez, A.G.; Rios, J.J.; Sanz, C. Aroma of virgin olive oil: Biogenesis of the green odor notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R.; Ríos, J.J. Dynamic headspace gas chromatographic method for determining volatiles in virgin olive oil. J. Chromatogr. A 1994, 68, 455–462. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with the volatile compound from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Salas, J.; Williams, M.; Harwood, J.L.; Sanchez, J. Lipoxygenase activity in olive (Olea europaea L.) fruit. J. Am. Oil Chem. Soc. 1999, 76, 1163–1169. [Google Scholar] [CrossRef]
- Salas, J.; Sanchez, J. Hydroperoxide lyase from olive (Olea europaea L.) fruits. Plant Sci. 1999, 143, 19–26. [Google Scholar] [CrossRef]
- Salas, J.J.; Sanchez, J. Alcohol dehydrogenases from olive (Olea europaea) fruit. Phytochemistry 1998, 48, 35–40. [Google Scholar] [CrossRef]
- Salas, J.J. Characterization of alcohol acyltransferase from olive fruit. J. Agric. Food Chem. 2004, 52, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W.; Grove, M.J.; Salch, Y.P. Enzymic pathway to ethyl vinyl ketone and 2-pentenal in soybean preparations. J. Agric. Food Chem. 1996, 44, 882–886. [Google Scholar] [CrossRef]
- Fisher, A.J.; Grimes, H.D.; Fall, R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry 2003, 62, 159–163. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Pérez, A.G.; Sanz, C. Cultivar differences on nonesterified polyunsaturated fatty acid as a limiting factor for biogenesis of virgin olive oil aroma. J. Agric. Food Chem. 2007, 55, 7869–7873. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiz, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Synthesis of volatile compounds of virgin olive oil is limited by the lipoxygenase activity load during the oil extraction process. J. Agric. Food Chem. 2012, 60, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Bedoukian, P.Z. The seven primary hexenols and their olfactory characteristics. J. Agric. Food Chem. 1971, 19, 1111–1114. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Horino, H.; Inokuchi, K.I. Odor-structure relationships in n-hexanols and n-hexenales. Z. Naturforsch. 1992, 47, 183–189. [Google Scholar]
- Aparicio, R.; Morales, M.T. Sensory wheels: A statistical technique for comparing QDA panels Application to virgin olive oil. J. Sci. Food Agric. 1995, 67, 247–257. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation 640/2008/EC. Off. J. Eur. Union 2008, L178, 11–16. [Google Scholar]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Belaj, A.; Dominguez-Garcia, M.C.; Atienza, S.G.; Martin-Urdiroz, N.; de la Rosa, R.; Satovic, Z.; Martin, A.; Kilian, A.; Trujillo, I.; Valpuesta, C.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- León, L.; Beltrán, G.; Aguilera, M.P.; Rallo, L.; Barranco, D.; de la Rosa, R. Oil composition of advanced selections from an olive breeding program. Eur. J. Lipid Sci. Technol. 2011, 113, 870–875. [Google Scholar] [CrossRef]
- Rjiba, I.; Gazzah, N.; Dabbou, S.; Hammami, M. Evaluation of virgin olive oil minor compounds in progenies of controlled crosses. J. Food Biochem. 2011, 35, 1413–1423. [Google Scholar] [CrossRef]
- Lavee, S. Aims, methods and advances in breeding of new olive (Olea europaea L.) cultivars. Acta Hortic. 1989, 286, 23–40. [Google Scholar] [CrossRef]
- Martinez, J.M.; Munoz, E.; Alba, J.; Lanzon, A. Report about the use of the ‘Abencor’ analyser. Grasas Aceites 1975, 26, 379–385. [Google Scholar]
- Perez, A.G.; de la Rosa, R.; Pascual, M.; Sanchez-Ortiz, A.; Romero-Segura, C.; Leon, L.; Sanz, C. Assessment of volatile compound profiles and the deduced sensory significance of virgin olive oils from the progeny of Picual × Arbequina cultivars. J. Chromatogr. A 2016, 1428, 305–315. [Google Scholar] [CrossRef] [PubMed]
- El Riachy, M.; Priego-Capote, F.; Rallo, L.; Luque de Castro, M.D.; León, L. Phenolic profile of virgin olive oil from advanced breeding selections. Span. J. Agric. Res. 2012, 10, 443–453. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile composition. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of virgin olive oils with different flavor profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Angerosa, F.; Camera, L.; d’Alessandro, N.; Mellerio, G. Characterization of seven new hydrocarbon compounds present in the aroma of virgin olive oils. J. Agric. Food Chem. 1998, 46, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Lanza, B.; Marsilio, V. Biogenesis of “fusty” defect in virgin olive oils. Grasas Aceites 1996, 47, 142–150. [Google Scholar] [CrossRef]
- García-Vico, L.; Belaj, A.; Sanchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile compound profiling by HS-SPME/GC-MS-FID of a core olive cultivar collection as a tool for aroma improvement of virgin olive oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortiz, A.; Romero-Segura, C.; Gazda, V.E.; Graham, I.A.; Sanz, C.; Perez, A.G. Factors limiting the synthesis of virgin olive oil volatile esters. J. Agric. Food Chem. 2012, 60, 1300–1307. [Google Scholar] [CrossRef] [PubMed]




| No. | Cultivar | No. | Cultivar |
|---|---|---|---|
| 1 | Abbadi Abou Gabra | 32 | Lastovka |
| 2 | Aggezi Shami | 33 | Lechín de Sevilla |
| 3 | Agouromanakolia | 34 | Lentisca |
| 4 | Amygdalolia Nana | 35 | Levantinka |
| 5 | Arbosana | 36 | Maurino |
| 6 | Argudell | 37 | Merhavia |
| 7 | Azapa o Arauco | 38 | Negrinha |
| 8 | Blanqueta | 39 | Ouslati |
| 9 | Blanqueta-48 | 40 | Palomar |
| 10 | Borriolenca | 41 | Patronet |
| 11 | Bosana | 42 | Pecoso |
| 12 | Canetera | 43 | Pequeña de Casas Ibañez |
| 13 | Caninese | 44 | Picholine |
| 14 | Chorreao de Montefrío | 45 | Picholine Marroquí |
| 15 | Cipresino | 46 | Rapasayo |
| 16 | Coratina | 47 | Sabatera |
| 17 | Corbella | 48 | Shami |
| 18 | Cornicabra | 49 | Torcio de Cabra |
| 19 | Curivell | 50 | Ulliri i Kuq |
| 20 | Elmacik | 51 | Vallesa |
| 21 | Figueretes | 52 | Vaneta |
| 22 | Galega Vulgar | 53 | Vera |
| 23 | Haouzia | 54 | Verde Verdelho |
| 24 | Istarska Bjelica | 55 | Verdial de Badajoz |
| 25 | Jaropo | 56 | Verdial de Vélez-Málaga |
| 26 | Joanenca | 57 | Vinyols |
| 27 | Kaesi | 58 | Wardan |
| 28 | Kalokerida | 59 | Yun Gelebi |
| 29 | Kan Celebi | 60 | Zaity |
| 30 | Klon-1081 | 61 | Zalmati |
| 31 | Kolybada |
| Volatile Compound | Code | Min | Max | Mean | % cv OAV > 1 | |
|---|---|---|---|---|---|---|
| C6/LnA aldehydes | (E)-hex-3-enal | 6C-1 | 116 | 924 | 371 | 25 |
| (Z)-hex-3-enal | 6C-2 | 91 | 4598 | 884 | 100 | |
| (Z)-hex-2-enal | 6C-3 | 121 | 1290 | 646 | 80 | |
| (E)-hex-2-enal | 6C-4 | 4705 | 65,029 | 27,190 | 100 | |
| C6/LnA alcohols | (E)-hex-3-enol | 6C-5 | 0 | 836 | 35 | 0 |
| (Z)-hex-3-enol | 6C-6 | 45 | 3577 | 581 | 11 | |
| (E)-hex-2-enol | 6C-7 | 43 | 3293 | 252 | 0 | |
| C6/LA aldehyde | hexanal | 6C-8 | 227 | 4451 | 1037 | 89 |
| C6/LA alcohol | hexan-1-ol | 6C-9 | 13 | 1463 | 314 | 26 |
| C5/LnA carbonyls | pent-1-en-3-one | 5C-1 | 126 | 1615 | 644 | 100 |
| (Z)-pent-2-enal | 5C-2 | 8 | 175 | 57 | 0 | |
| (E)-pent-2-enal | 5C-3 | 45 | 226 | 105 | 0 | |
| C5/LnA alcohols | pent-1-en-3-ol | 5C-4 | 80 | 634 | 257 | 10 |
| (Z)-pent-2-en-1-ol | 5C-5 | 15 | 190 | 54 | 0 | |
| (E)-pent-2-en-1-ol | 5C-6 | 187 | 1564 | 510 | 90 | |
| Pentene dimers | pentene dimer-1 | 5C-7 | 77 | 1195 | 457 | 0 |
| pentene dimer-2 | 5C-8 | 65 | 1036 | 360 | 0 | |
| pentene dimer-3 | 5C-9 | 346 | 4840 | 1812 | 0 | |
| pentene dimer-4 | 5C-10 | 240 | 6363 | 2011 | 0 | |
| pentene dimer-5 | 5C-11 | 0 | 4513 | 1282 | 0 | |
| pentene dimer-6 | 5C-12 | 121 | 3909 | 1260 | 0 | |
| pentene dimer-7 | 5C-13 | 0 | 2847 | 628 | 0 | |
| C5/LA carbonyls | pentan-3-one | 5C-14 | 17 | 270 | 91 | 0 |
| pentanal | 5C-15 | 12 | 233 | 48 | 0 | |
| C5/LA alcohol | pentan-1-ol | 5C-16 | 0 | 26 | 5 | 0 |
| LOX esters | hexyl acetate | E-1 | 5 | 6632 | 639 | 16 |
| (E)-hex-2-en-1-yl acetate | E-2 | 8 | 1620 | 66 | 3 | |
| (Z)-hex-3-en-1-yl acetate | E-3 | 0 | 8696 | 1139 | 52 | |
| non-LOX esters | methyl acetate | E-4 | 6 | 42 | 19 | 0 |
| ethyl acetate | E-5 | 5 | 354 | 24 | 0 | |
| methyl hexanoate | E-6 | 6 | 128 | 29 | 0 | |
| ethyl hexanoate | E-7 | 0 | 359 | 45 | 0 | |
| BC aldehydes | 3-methyl-butanal | BC-1 | 6 | 351 | 43 | 100 |
| 2-methyl-butanal | BC-2 | 5 | 342 | 27 | 100 | |
| BC alcohol | 2-methyl-butan-1-ol | BC-3 | 6 | 159 | 36 | 0 |
| Terpene | limonene | T-1 | 0 | 743 | 59 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, C.; Belaj, A.; Sánchez-Ortiz, A.; Pérez, A.G. Natural Variation of Volatile Compounds in Virgin Olive Oil Analyzed by HS-SPME/GC-MS-FID. Separations 2018, 5, 24. https://doi.org/10.3390/separations5020024
Sanz C, Belaj A, Sánchez-Ortiz A, Pérez AG. Natural Variation of Volatile Compounds in Virgin Olive Oil Analyzed by HS-SPME/GC-MS-FID. Separations. 2018; 5(2):24. https://doi.org/10.3390/separations5020024
Chicago/Turabian StyleSanz, Carlos, Angjelina Belaj, Araceli Sánchez-Ortiz, and Ana G. Pérez. 2018. "Natural Variation of Volatile Compounds in Virgin Olive Oil Analyzed by HS-SPME/GC-MS-FID" Separations 5, no. 2: 24. https://doi.org/10.3390/separations5020024
APA StyleSanz, C., Belaj, A., Sánchez-Ortiz, A., & Pérez, A. G. (2018). Natural Variation of Volatile Compounds in Virgin Olive Oil Analyzed by HS-SPME/GC-MS-FID. Separations, 5(2), 24. https://doi.org/10.3390/separations5020024