Recent Advances in Molecularly Imprinted Membranes for Sample Treatment and Separation
Abstract
:1. Introduction
2. General Aspects of MIMs and Preparation
2.1. Fundamental Aspects in Membrane Separation
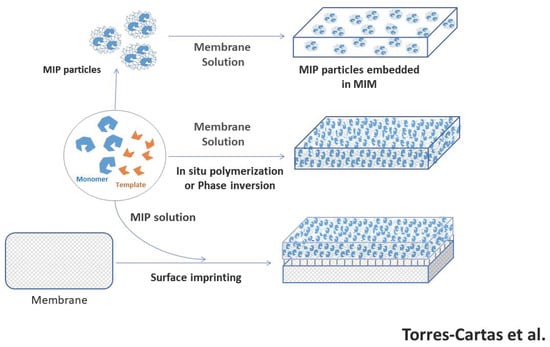
2.2. Types of Strategies to Synthesize MIMs
3. Use of MIMs in Sample Preparation
3.1. Pharmaceutical and Clinical Applications
3.2. Food Applications
3.3. Environmental Applications
4. Use of MIMs in Separation Processes
4.1. Pharmaceutical and Food Applications
4.2. Environmental Applications
| Analyte(s) | Template/Monomer/Crosslinker/Solvent | Substrate | Imprinting Technique | Matrix | Determination Technique | IF/SF | PSF | Reference |
|---|---|---|---|---|---|---|---|---|
| 2-AP | 2-AP/-/-/DMAc:H2O | PBI | Phase inversion | - | HPLC-UV | 4.1/5.5 | 3.9 | [23] |
| Paclitaxel | Paclitaxel/-/-/DMF | PSf | Phase inversion | Yew tree extract | HPLC-UV | 2.28/- | - | [52] |
| Matrine | Matrine/MAA/EGDMA/H2O:MeOH | CMPSf | Surface grafting | - | UV | 140/4.85 | 5.9 | [50] |
| Ars | Ars/Am/EGDMA/Pyridine | PVDF | Surface grafting | - | HPLC-UV | 14.9/2.04 | 5.6 | [51] |
| Propranolol | Propranolol/MAA/MBA/H2O | Br-Ag-pDA@SiO2-based composite PVDF | Surface grafting | - | HPLC-UV | 4.85/2.70–3.24 | 5.52–6.71 | [53] |
| Propranolol | Propranolol/MAA, AM/MBA/EtOH | GO/TiO2-based composite RC | Surface grafting | - | HPLC-UV | 4.26/ 3.0–3.2 | 11.35–13.66 | [54] |
| Ibuprofen | Ibuprofen/APTES/TEOS/EtOH | pDA@GO-based composite PVDF | Surface grafting | - | UV | 4.38/3.51–3.91 | 6.55–6.63 | [55] |
| Ibuprofen | Ibuprofen/APTES/TEOS/EtOH | pDA@TiO2-based composite PVDF | Surface grafting | - | UV | 4.68/3.25–3.66 | 4.42–4.46 | [56] |
| Tetracycline | Tetracycline/MAA/EGDMA/H2O | Ag/pDA@Al2O3 ceramic membrane | Surface grafting | - | UV | 2.64/3.27–3.60 | 5.95–6.15 | [57] |
| Phenol | Phenol/4-VP/DVB/MeOH | SAN | In situ polymerization (embedded membrane) | - | UV | -/ 5.54–5.68 | - | [58] |
| Cholesterol | Cholesterol/MTrp, HEMA/EGDMA/MeOH | HEMA | In situ polymerization (embedded membrane) | Intestinal mimic solution | HPLC-UV | -/- 2.04–2.39 | - | [59] |
| Cholesterol | Cholesterol/MAA/EGDMA/ACN | - | In situ polymerization | Blood | UV | - | - | [60] |
| D-Tryptophan | D-Tryptophan/-/CaCl2/H2O | SAg | Phase inversion | - | HPLC-UV | - | - | [61] |
| L-glu | L-glu/DMAEMA/MBA/DMF-H2O | CMPSf | Surface grafting | - | UV | 1.44/7.52 | 3.25 | [62] |
| BSA | (pDA)-BSA/-/-/H2O | CS/PVP/MWCNTs | Phase inversion | Bovine blood | Fluorescence | 2.80/1.73-2.14 | - | [63] |
| BSA | BSA/ HEMA-MAP/EGDMA/PBS | - | In situ polymerization | - | UV | 3.74/1.12–1.34 | - | [64] |
| BSA | BSA/VAMIN/MBA/PBS | - | In situ cryo-polymerization | Blood | UV | 2.37/1.33–2.35 | 1.73 | [65] |
| BSA; Lys | BSA or Lys/MMA/EGDMA/NMP | CA | Surface grafting | Cell broth | HPLC-UV | 2.39–4.23/ - | 4–9 | [66] |
| Kaempferol | Kaempferol/4-VP/EGDMA/ACN | PPSU | Phase inversion | - | UV | 4.12 | - | [68] |
| Quercetin | Quercetin/HEMA-MAH/EGDMA/isopropyl alcohol | - | In situ polymerization | - | UV | 30.6/- | - | [69] |
| Polyphenols | Quercetin/AN/EGDMA/ACN | CA | In situ polymerization (embedded membrane) | Lemon, orange and onion peels | HPLC-UV | - | - | [22] |
| Analyte(s) | Template/Monomer/Crosslinker/Solvent | Substrate | Imprinting Technique | Matrix | Determination Technique | IF/SF | PSF | Referemce |
|---|---|---|---|---|---|---|---|---|
| Pb2+ | Pb(NO2)3/PAA/GLA/H2O | PVA | In situ polymerization (embedded membrane) | - | ICP-OES | 1.25/ 70 | - | [70] |
| Li+ | Li+/12C4, MAA, EGDMA/ACN | PES | Surface grafting | - | ICP-OES | 2.55/1.85–2.07 | 7.39–9.86 | [71] |
| Eu3+ | Eu3+/NIPAm, Am/EGDMA/ACN | RCM | Surface grafting | - | ICP-OES | 4.09/1.45–1.82 | 3.34–3.82 | [72] |
| 2,4-D | 2,4-D/MAA/TRIM/DMAc | PSf | Phase inversion | - | UV | -/12.96 | 1.7 | [73] |
| 2,4-D | 2,4-D/N-vinylimidazole/EGDMA/MeOH:water | - | In situ polymerization | Water | UV | 1.1–3.1/ 2.35–2.74 | - | [74] |
| Phenol | Phenol/MAA/TRIM/ACN | PSf | Phase inversion | - | UV | 1.08/3.57 | - | [75] |
| BPA | BPA/MMA/EGDMA/n-octane | PP | Plasma-induced grafting | - | UV | 2.9–9/- | 32.2-78.3 | [76] |
| 4-Nitrophenol | 4-Nitrophenol/-/GLA/PEG | CS | In situ polymerization | Water | HPLC-UV | 1.52/2.46–16.19 | - | [77] |
| m-Cresol | m-Cresol/APTES/TEOS/EtOH | RCM | Surface grafting | - | HPLC-UV | 3.07/4.41–5.41 | 13.17–15.44 | [78] |
| Xylene isomers | 1,2-dihydroxybenzene /-/LiCl/DMAc | CM | Phase inversion | - | UV | -/4.24–7.15 | - | [80] |
| PAHs | Anthracene/MAA/EGDMA/ACN | CA | Phase inversion | - | HPLC-UV | -/5–18.8 | - | [81] |
| Naphthalene | Naphthalene/-/DMF | PSf | Phase inversion | Wastewater streams | UV | 1.28/2.27 | - | [79] |
| PCBs | Anthracene/MAA/EGDMA/ACN | PSf | Phase inversion | Water | CG-TOF-MS | - | 8.23–10.3 | [82] |
| DBTu | DBTu/PMMA/CHCl3 | - | In situ polymerization | - | UV | 2.60–3.0/- | - | [83] |
| RhB | RhB/MAA/EGDMA/ACN | LDH-based material | Surface grafting | Water | UV | 2.83/8.8–27.3 | - | [84] |
| MB | MB/MAA/MBA/THF | PSf | Phase inversion | - | UV | - | 2.83–2.91 | [85] |
| Norfloxacin | Norfloxacin/Am/EGDMA/EtOH | TiO2@GO/PVDF | Surface grafting | Water | UV | 5.73/5.45–7.35 | 6.25 | [86] |
| TC | TC/MAA, Am/EDGMA/EtOH | ACNPs@ CA/CS | Surface grafting | Water | HPLC-UV | 3.3–3.6/3.4 | 2.4 | [87] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922. [Google Scholar] [CrossRef]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowicz, P.; Noworyta, K.; D’Souza, F.; Kutner, W. Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. TrAC Trends Anal. Chem. 2019, 118, 793–809. [Google Scholar] [CrossRef]
- Sari, E.; Üzek, R.; Merkoçi, A. Paper Based Photoluminescent Sensing Platform with Recognition Sites for Tributyltin. ACS Sens. 2019, 4, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly imprinted polymers for the identification and separation of chiral drugs and biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Gilart, N.; Borrull, F.; Fontanals, N.; Marcé, R.M. Selective materials for solid-phase extraction in environmental analysis. Trends Environ. Anal. Chem. 2014, 1, e8–e18. [Google Scholar] [CrossRef]
- Schirhagl, R. Bioapplications for molecularly imprinted polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Recent Developments in Selective Materials for Solid Phase Extraction. Chromatographia 2019, 82, 1171–1189. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC-Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.-O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Boysen, R.I.; Schwarz, L.J.; Nicolau, D.V.; Hearn, M.T.W. Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci. 2017, 40, 314–335. [Google Scholar] [CrossRef]
- Meng, L.; Turner, A.P.F.; Mak, W.C. Soft and flexible material-based affinity sensors. Biotechnol. Adv. 2020, 39, 107398. [Google Scholar] [CrossRef]
- Trotta, F.; Biasizzo, M.; Caldera, F. Molecularly imprinted membranes. Membranes 2012, 2, 440–477. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, L.; Fu, C. The Recognizing Mechanism and Selectivity of the Molecularly Imprinting Membrane. In Molecularly Imprinted Catalysts; Elsevier: Amsterdam, The Netherlands, 2016; pp. 159–182. ISBN 9780128013014. [Google Scholar]
- Piletsky, S.A.; Panasyuk, T.L.; Piletskaya, E.V.; Nicholls, I.A.; Ulbricht, M. Receptor and transport properties of imprinted polymer membranes - A review. J. Memb. Sci. 1999, 157, 263–278. [Google Scholar] [CrossRef]
- Ulbricht, M. Membrane separations using molecularly imprinted polymers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 804, 113–125. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef]
- Mansour, M.S.M.; Abdel-Shafy, H.I.; Mehaya, F.M.S. Valorization of food solid waste by recovery of polyphenols using hybrid molecular imprinted membrane. J. Environ. Chem. Eng. 2018, 6, 4160–4170. [Google Scholar] [CrossRef]
- Székely, G.; Valtcheva, I.B.; Kim, J.F.; Livingston, A.G. Molecularly imprinted organic solvent nanofiltration membranes—Revealing molecular recognition and solute rejection behaviour. React. Funct. Polym. 2015, 86, 215–224. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Memb. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- He, Z.; Meng, M.; Yan, L.; Zhu, W.; Sun, F.; Yan, Y.; Liu, Y. Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ([BMIM] Cl) for selective adsorption of salicylic acid from industrial wastewater. Sep. Purif. Technol. 2015, 145, 63–74. [Google Scholar] [CrossRef]
- Xu, X.; Wan, L.; Huang, X. Functionalization Methods for Membrane Surfaces. In Surface Engineering of Polymer Membranes; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Yao, R.; Yu, Z.; Wu, M.; Yu, H. Preparation and evaluation of molecularly imprinted membrane of teicoplanin. Anal. Methods 2018, 10, 5416–5422. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.W.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, M.; Cui, J.; Yan, Y.; Li, C. A Multiple-Functional Ag/SiO2/Organic Based Biomimetic Nanocomposite Membrane for High-Stability Protein Recognition and Cell Adhesion/Detachment. Adv. Funct. Mater. 2015, 25, 5823–5832. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, M.; Liu, X.; Lv, P.; Cui, J.; Meng, M.; Dai, J.; Yan, Y.; Li, C. Accelerating the design of multi-component nanocomposite imprinted membranes by integrating a versatile metal—organic methodology. Green Chem. 2015, 17, 3338–3349. [Google Scholar] [CrossRef]
- Beigzadeh, Z.; Golbabaei, F.; Khadem, M.; Omidi, F.; Someah, M.S.; Shahtaheri, S.J. Development of Molecularly Imprinted Membranes for Selective Determination of Urinary Ultra-Trace 5-Fluorouracil as Antineoplastic Drug Used in Chemotherapy. Macromol. Res. 2020, 28, 390–399. [Google Scholar] [CrossRef]
- Yu, H.; Yao, R.; Shen, S. Development of a novel assay of molecularly imprinted membrane by design-based gaussian pattern for vancomycin determination. J. Pharm. Biomed. Anal. 2019, 175, 112789. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-adergani, B.; Abdel-Rehim, M. A new strategy for surface modification of polysulfone membrane by in situ imprinted sol–gel method for the selective separation and screening of L-Tyrosine as a lung cancer biomarker. Analyst 2015, 140, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, L.; Wang, Y.; Huang, X.; Xu, J.; Lu, J.; Zhang, M.; Xu, W. Molecularly Imprinted Membrane Electrospray Ionization for Direct Sample Analyses. Anal. Chem. 2017, 89, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.S.; Carvalho, T.C.; Romão, W.; Vaz, B.G.; Chaves, A.R. Paper Spray Tandem Mass Spectrometry Based on Molecularly Imprinted Polymer Substrate for Cocaine Analysis in Oral Fluid. J. Am. Soc. Mass Spectrom. 2018, 29, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, J.; Tabernero, M.J.; Bermejo, A.M.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Porous membrane-protected molecularly imprinted polymer micro-solid-phase extraction for analysis of urinary cocaine and its metabolites using liquid chromatography—Tandem mass spectrometry. Anal. Chim. Acta 2015, 898, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, J.; García-Carballal, S.; Cabarcos, P.; Tabernero, M.J.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Determination of cocaine and its metabolites in plasma by porous membrane-protected molecularly imprinted polymer micro-solid-phase extraction and liquid chromatography—tandem mass spectrometry. J. Chromatogr. A 2016, 1451, 15–22. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Odoardi, S.; Bermejo, A.M.; Bermejo-Barrera, P.; Romolo, F.S.; Moreda-Piñeiro, A.; Strano-Rossi, S. Development of a micro-solid-phase extraction molecularly imprinted polymer technique for synthetic cannabinoids assessment in urine followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2018, 1550, 8–20. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Odoardi, S.; Bermejo, A.M.; Bermejo-Barrera, P.; Romolo, F.S.; Moreda-Piñeiro, A.; Strano-Rossi, S. HPLC-MS/MS combined with membrane-protected molecularly imprinted polymer micro-solid-phase extraction for synthetic cathinones monitoring in urine. Drug Test. Anal. 2019, 11, 33–44. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Nakajima, L.; Kobayashi, T. Preparation and Characterization of Hybrid Molecularly Imprinted Polymer Membranes for the Determination of Citrinin in Rice. Sains Malays. 2019, 48, 1661–1670. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-adergani, B. Fabrication of a novel electrospun molecularly imprinted nanomembrane coupled with high-performance liquid chromatography for the selective separation and determination of acesulfame. J. Sep. Sci. 2015, 38, 1372–1379. [Google Scholar] [CrossRef]
- Akbari-adergani, B.; Sadeghian, G.H.; Alimohammadi, A.; Esfandiari, Z. Integrated photografted molecularly imprinted polymers with a cellulose acetate membrane for the extraction of melamine from dry milk before HPLC analysis. J. Sep. Sci. 2017, 40, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Rodrigues, M.F.; Chaves, A.R.; Vaz, B.G. Molecularly imprinted polymer (MIP) membrane assisted direct spray ionization mass spectrometry for agrochemicals screening in foodstuffs. Talanta 2018, 178, 507–514. [Google Scholar] [CrossRef]
- Jayasinghe, G.D.T.M.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ultrasound assisted combined molecularly imprinted polymer for the selective micro-solid phase extraction and determination of aflatoxins in fish feed using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1609, 460431. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Bai, M.; Da, Z.; Cui, Y.; Li, B.; Pan, J. Selective recognition of salicylic acid employing new fluorescent imprinted membrane functionalized with poly(amidoamine) (PAMAM)-encapsulated Eu(TTA)3phen. J. Lumin. 2019, 208, 24–32. [Google Scholar] [CrossRef]
- Rozaini, M.N.H.; Semail, N.; Saad, B.; Kamaruzaman, S.; Abdullah, W.N.; Rahim, N.A.; Miskam, M.; Loh, S.H.; Yahaya, N. Molecularly imprinted silica gel incorporated with agarose polymer matrix as mixed matrix membrane for separation and preconcentration of sulfonamide antibiotics in water samples. Talanta 2019, 199, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Chianella, I.; Da Ponte, G.; Paulussen, S.; Gaeta, S.; Tothill, I.E. Development of functionalized nanostructured polymeric membranes for water purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar] [CrossRef]
- Ncube, S.; Lekoto, G.; Cukrowska, E.; Chimuka, L. Development and optimisation of a novel three-way extraction technique based on a combination of Soxhlet extraction, membrane assisted solvent extraction and a molecularly imprinted polymer using sludge polycyclic aromatic hydrocarbons as model compounds. J. Sep. Sci. 2018, 41, 918–928. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Li, Y. Designing and preparation of novel alkaloid-imprinted membrane with grafting type and its molecular recognition characteristic and permselectivity. Mater. Sci. Eng. C 2016, 66, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Wu, Y.; Meng, M.; Lu, J.; Wang, C.; Zhao, J.; Yan, Y. Bio-inspired synthesis of molecularly imprinted nanocomposite membrane for selective recognition and separation of artemisinin. J. Appl. Polym. Sci. 2016, 133, 43405. [Google Scholar] [CrossRef]
- Ghasemi, S.; Nematollahzadeh, A. Molecularly imprinted ultrafiltration polysulfone membrane with specific nano-cavities for selective separation and enrichment of paclitaxel from plant extract. React. Funct. Polym. 2018, 126, 9–19. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, J.; Wang, C.; Lu, J.; Meng, M.; Dai, X.; Yan, Y.; Li, C. A novel approach toward fabrication of porous molecularly imprinted nanocomposites with bioinspired multilevel internal domains: Application to selective adsorption and separation membrane. Chem. Eng. J. 2016, 306, 492–503. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Meng, M.; Lv, P.; Liu, X.; Yan, Y. Fabrication and evaluation of GO/TiO2-based molecularly imprinted nanocomposite membranes by developing a reformative filtering strategy: Application to selective adsorption and separation membrane. Sep. Purif. Technol. 2019, 212, 245–254. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Chen, L.; Yan, L.; Zhou, S.; Zhang, Q.; Li, C.; Yan, Y.; Li, H. Bioinspired synthesis of pDA@GO-based molecularly imprinted nanocomposite membranes assembled with dendrites-like Ag microspheres for high-selective adsorption and separation of ibuprofen. J. Memb. Sci. 2018, 553, 151–162. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y. Fabrication and evaluation of bioinspired pDA@TiO2-based ibuprofen-imprinted nanocomposite membranes for highly selective adsorption and separation applications. New J. Chem. 2020, 44, 10703–10712. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Cui, J.; Meng, M.; Dai, J.; Li, C.; Yan, Y. Bioinspired synthesis of high-performance nanocomposite imprinted membrane by a polydopamine-assisted metal-organic method. J. Hazard. Mater. 2017, 323, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Meng, M.; Yan, L.; He, Z.; Yan, Y.; Liu, Y.; Liu, S. Fabrication of ordered microporous styrene-acrylonitrile copolymer blend imprinted membranes for selective adsorption of phenol from salicylic acid using breath figure method. J. Appl. Polym. Sci. 2015, 132, 42350. [Google Scholar] [CrossRef]
- Odabaşı, M.; Uzun, L.; Baydemir, G.; Aksoy, N.H.; Acet, Ö.; Erdönmez, D. Cholesterol imprinted composite membranes for selective cholesterol recognition from intestinal mimicking solution. Colloids Surf. B Biointerfaces 2018, 163, 266–274. [Google Scholar] [CrossRef]
- Niesa, J.; Ulianas, A. Design and characterization of membrane molecularly imprinted polymer (MIP) as cholesterol absorbent. J. Phys. Conf. Ser. 2020, 1481, 012031. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, K.; Mao, Y.; Chai, W.; Wang, N.; Ren, Z. Green preparation of d-tryptophan imprinted self-supported membrane for ultrahigh enantioseparation of racemic tryptophan. RSC Adv. 2016, 6, 109992–110000. [Google Scholar] [CrossRef]
- Gao, B.; Cui, K.; Li, Y. Preparation of molecule imprinted membrane of single enantiomer of amino acid with an innovative strategy and study on its chiral recognition and resolution properties. J. Chem. Technol. Biotechnol. 2017, 92, 1566–1576. [Google Scholar] [CrossRef]
- Luo, Z.; Du, W.; Guo, P.; Zheng, P.; Chang, R.; Wang, J.; Zeng, A.; Chang, C.; Fu, Q. A porous hybrid imprinted membrane for selectively anchoring target proteins from a complex matrix. RSC Adv. 2015, 5, 72610–72620. [Google Scholar] [CrossRef]
- Demir, E.F.; Özçalışkan, E.; Karakaş, H.; Uygun, M.; Aktaş Uygun, D.; Akgöl, S.; Denizli, A. Synthesis and characterization of albumin imprinted polymeric hydrogel membranes for proteomic studies. J. Biomater. Sci. Polym. Ed. 2018, 29, 2218–2236. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-P.; Zhang, F.-Y.; Yang, X.-M.; Zhang, X.-H.; Cao, Y.-H.; Peng, H.-L. Preparation of a novel supermacroporous molecularly imprinted cryogel membrane with a specific ionic liquid for protein recognition and permselectivity. J. Appl. Polym. Sci. 2018, 135, 46740. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Tong, W.; Sankarakumar, N.; Yin, M.; Wu, D.; Duan, X. Specific purification of a single protein from a cell broth mixture using molecularly imprinted membranes for the biopharmaceutical industry. RSC Adv. 2019, 9, 23425–23434. [Google Scholar] [CrossRef] [Green Version]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, P.; Yun, Y. Preparing molecularly imprinted membranes by phase inversion to separate kaempferol. Polym. Adv. Technol. 2017, 28, 373–378. [Google Scholar] [CrossRef]
- Türkcan, C.; Somtürk, B.; Özdemir, N.; Özel, M.; Çatalkaya, R.; Aktaş Uygun, D.; Uygun, M.; Akgöl, S. Quercetin adsorption with imprinted polymeric materials. J. Biomater. Sci. Polym. Ed. 2019, 30, 947–960. [Google Scholar] [CrossRef]
- He, J.; Liu, A.; Paul Chen, J. Introduction and demonstration of a novel Pb(II)-imprinted polymeric membrane with high selectivity and reusability for treatment of lead contaminated water. J. Colloid Interface Sci. 2015, 439, 162–169. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.; Zhang, Q.; Wu, Y.; Cui, J.; Li, C.; Wang, L.; Yan, Y. Multilayered ion-imprinted membranes with high selectivity toward Li + based on the synergistic effect of 12-crown-4 and polyether sulfone. Appl. Surf. Sci. 2018, 427, 931–941. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Y.; Lin, X.; Gao, J.; Dong, H.; Chen, L.; Qin, Y.; Wang, L.; Yan, Y. Anti-fouling and thermosensitive ion-imprinted nanocomposite membranes based on grapheme oxide and silicon dioxide for selectively separating europium ions. J. Hazard. Mater. 2018, 353, 244–253. [Google Scholar] [CrossRef]
- Kashani, T.; Jahanshahi, M.; Rahimpour, A.; Peyravi, M. Nanopore Molecularly Imprinted Polymer Membranes for Environmental Usage: Selective Separation of 2,4-Dichlorophenoxyacetic Acid as a Toxic Herbicide from Water. Polym. Plast. Technol. Eng. 2016, 55, 1700–1712. [Google Scholar] [CrossRef]
- Söylemez, M.A.; Güven, O. Radiation induced in-situ synthesis of membranes for removal of 2,4-dichlorophenoxy acetic acid from real water samples. Radiat. Phys. Chem. 2020, 171, 108708. [Google Scholar] [CrossRef]
- Ashrafian, S.; Ataei, S.A.; Jahanshahi, M. Novel composite membranes embedded with molecularly imprinted porous polymeric nanospheres for targeted phenol. Polym. Adv. Technol. 2016, 27, 789–804. [Google Scholar] [CrossRef]
- Wolska, J.; Smolinska-Kempisty, K. Polypropylene prefilters with surface imprinted layer. Sep. Purif. Technol. 2017, 174, 89–96. [Google Scholar] [CrossRef]
- Di Bello, M.P.; Mergola, L.; Scorrano, S.; Del Sole, R. Towards a new strategy of a chitosan-based molecularly imprinted membrane for removal of 4-nitrophenol in real water samples. Polym. Int. 2017, 66, 1055–1063. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, W.; Yan, J.; Cui, J.; Ma, F.; Gao, J.; Lu, J.; Yu, C.; Yan, M. Multilevel mineral-coated imprinted nanocomposite membranes for template-dependent recognition and separation: A well-designed strategy with PDA/CaCO3-based loading structure. J. Colloid Interface Sci. 2020, 575, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Nematollahzadeh, A. Molecularly imprinted polymer membrane for the removal of naphthalene from petrochemical wastewater streams. Adv. Polym. Technol. 2018, 37, 2288–2293. [Google Scholar] [CrossRef]
- Zheng, H.; Yoshikawa, M. Molecularly imprinted cellulose membranes for pervaporation separation of xylene isomers. J. Memb. Sci. 2015, 478, 148–154. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Sayour, H.E.; Mansour, M.S.M. Molecular imprinted membrane based on molecular imprinted nanoparticles polymer for separation of polycyclic aromatic hydrocarbons. Polym. Adv. Technol. 2016, 27, 724–732. [Google Scholar] [CrossRef]
- Mkhize, D.S.; Nyoni, H.; Quinn, L.P.; Mamba, B.B.; Msagati, T.A.M. Molecularly imprinted membranes (MIMs) for selective removal of polychlorinated biphenyls (PCBs) in environmental waters: Fabrication and characterization. Environ. Sci. Pollut. Res. 2017, 24, 11694–11707. [Google Scholar] [CrossRef]
- Mujahid, A.; Maryam, A.; Afzal, A.; Zafar Bajwa, S.; Hussain, T.; Imran Din, M.; Latif, U.; Irshad, M. Molecularly imprinted poly(methyl methacrylate)-nickel sulfide hybrid membranes for adsorptive desulfurization of dibenzothiophene. Sep. Purif. Technol. 2020, 237, 116453. [Google Scholar] [CrossRef]
- Lu, L.; Yue, X.; Lin, F.; Huang, F.; Zhang, B.; Lin, Z. Template-synthesized ultra-thin molecularly imprinted polymers membrane for the selective preconcentration of dyes. J. Mater. Chem. A 2015, 3, 10959–10968. [Google Scholar] [CrossRef]
- Melvin Ng, H.K.; Leo, C.P.; Abdullah, A.Z. Selective removal of dyes by molecular imprinted TiO2 nanoparticles in polysulfone ultrafiltration membrane. J. Environ. Chem. Eng. 2017, 5, 3991–3998. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Zhou, S.; Yan, L.; Dong, H.; Chen, L.; Meng, M.; Li, C.; Yan, Y. Molecularly imprinted nanocomposite membranes based on GO/PVDF blended membranes with an organic–inorganic structure for selective separation of norfloxacin. New J. Chem. 2017, 41, 14966–14976. [Google Scholar] [CrossRef]
- Xing, W.; Wu, Y.; Lu, J.; Lin, X.; Yu, C. Biomass-Based Synthesis of Green and Biodegradable Molecularly Imprinted Membranes for Selective Recognition and Separation of Tetracycline. Nano 2020, 15, 2050004. [Google Scholar] [CrossRef]


















| Analyte(s) | Template/Monomer/Crosslinker/Solvent | Substrate | Imprinting Technique | Matrix | Determination Technique | Recovery (%) | LOD (ng mL−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| Pharmaceutical and clinical | ||||||||
| 5-FU | 5-FU/MAA/EGDMA/MeOH:ACN | PET | Electrospinning | Urine | HPLC-UV | >93 | 0.023 | [32] |
| TE | TE/AAM/EGDMA/MeOH | Organic nylon PVDF; PP | Surface grafting | Serum | UV | >70.8 | - | [27] |
| VCM | VCM/AAM/EGDMA/MeOH | Organic nylon; PVDF; PP | Surface grafting | Serum | UV | >78 | - | [33] |
| L-Tys | L-Tys/γ-MPS/ACN | PSf | Surface grafting | Plasma | LC/MS/MS | >80 | 0.1 nmol L−1 | [34] |
| CLE MTX CPFX CPF | CLE or MTX or CPFX or CPF/MAA/EGDMA/CHCl3:MeOH | PVDF | Surface grafting | Urine Blood Milk Soil | ESI-MS | >91% | 0.02 (CLE) | [35] |
| Cocaine | Cocaine/MAA/EGDMA/H2O | Cellulose | Surface grafting | Oral fluid | PSI-MS | >100.5 | 0.27 | [36] |
| COC BZE CE EME | COC/EGDMA/DVB/ACN:TOL | PP | In situ polymerization (MIP particles enclosed in membrane) | Urine | HPLC-MS/MS | >97 | 0.05–0.5 | [37] |
| COC BZE CE EME | COC/EGDMA/DVB/ACN:TOL | PP | In situ polymerization (MIP particles enclosed in membrane) | Plasma | HPLC-MS/MS | >96 | 0.06–0.87 | [38] |
| Cannabinoids (JWH007;JWH015;JWH098) | JWH105/EGDMA/DVB/ACN:TOL | PP | In situ polymerization (MIP particles enclosed in membrane) | Urine | HPLC-MS/MS | >86 | 0.032–0.75 | [39] |
| Synthetic cathinones | Ethylone/EGDMA/DVB/ACN:TOL | PP | In situ polymerization (MIP particles enclosed in membrane) | Urine | HPLC-MS/MS | >92 | 0.14–1.02 | [40] |
| Food | ||||||||
| CIT | 1-naphthol/Methacryloyl chloride/DVB/Ace:H2O | PES | Phase inversion | Rice | HPLC-FD | >90 | 0.5 ng g−1 | [41] |
| Acesulfame | Acesulfame/TEPAM/ACN | Nylon 6 | Electrospinning | Beverages | HPLC-UV | >80 | 0.6 | [42] |
| Melamine | Melamine/MAA/EGDMA/ ACN:H2O | CA | Surface grafting | Milk | HPLC-UV | >89 | 7 | [43] |
| 2,4-D Diuron | 2,4,5-TD or monuron/MAA/ EGDMA/MeOH | CM | Surface grafting | Fresh fruit | PSI-MS | >92 | 0.17–0.6 | [44] |
| Aflatoxins (AFB1; AFB2; AFG1; AFG2) | DMC/MAA/DVB/ACN:TOL | PP | In situ polymerization (MIP particles enclosed in membrane) | Fish feed | UHPLC-MS-MS | >80 | 0.42–1.2 µg Kg−1 | [45] |
| Environmental | ||||||||
| SA | SA/CS/DMSO | CA | Phase inversion | Water | FD | - | 24000 | [46] |
| SMX SMM SDZ | SMX/APTES/TEOS/ACN | Agarose | In situ polymerization (encapsulated) | Water | HPLC-DAD | 80–96 | 0.06–0.17 | [47] |
| Diclofenac Metoprolol VCM | Diclofenac or metoprolol or VCM/NIPAm, AAc, TBAm /Bis /H2O | PVDF | Surface grafting | Water | HPLC-UV | 50.1–100 | 3.7–15 | [48] |
| PAHs | B[k]F+Indeno/p-vinylbenzene/EDGMA/DMF | PP | In situ polymerization (MIP particles enclosed in membrane) | Wastewater | GC-TOF/MS | 63–96 | 0.01–0.45 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Cartas, S.; Catalá-Icardo, M.; Meseguer-Lloret, S.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Recent Advances in Molecularly Imprinted Membranes for Sample Treatment and Separation. Separations 2020, 7, 69. https://doi.org/10.3390/separations7040069
Torres-Cartas S, Catalá-Icardo M, Meseguer-Lloret S, Simó-Alfonso EF, Herrero-Martínez JM. Recent Advances in Molecularly Imprinted Membranes for Sample Treatment and Separation. Separations. 2020; 7(4):69. https://doi.org/10.3390/separations7040069
Chicago/Turabian StyleTorres-Cartas, Sagrario, Mónica Catalá-Icardo, Susana Meseguer-Lloret, Ernesto F. Simó-Alfonso, and José Manuel Herrero-Martínez. 2020. "Recent Advances in Molecularly Imprinted Membranes for Sample Treatment and Separation" Separations 7, no. 4: 69. https://doi.org/10.3390/separations7040069
APA StyleTorres-Cartas, S., Catalá-Icardo, M., Meseguer-Lloret, S., Simó-Alfonso, E. F., & Herrero-Martínez, J. M. (2020). Recent Advances in Molecularly Imprinted Membranes for Sample Treatment and Separation. Separations, 7(4), 69. https://doi.org/10.3390/separations7040069