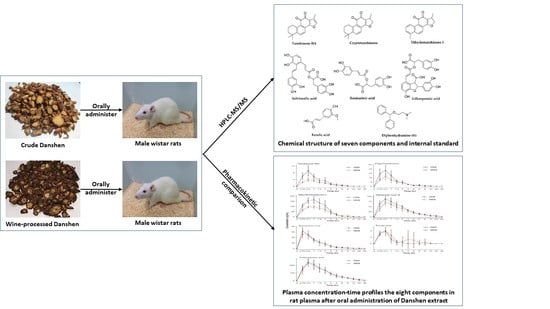
Simultaneous Determination of Seven Bioactive Constituents from Salvia miltiorrhiza in Rat Plasma by HPLC-MS/MS: Application to a Comparative Pharmacokinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Instruments, Liquid Chromatography, and Mass Spectrometry Conditions
2.3. Preparation of Calibration Standards, Internal Standard (IS), and Quality Control (QC) Samples
2.4. Preparation of Crude and Wine-Processed Danshen Extract
2.5. Preparation and Handling of Biological Samples
2.6. Method Validation
2.6.1. Selectivity
2.6.2. Linearity and Lower Limit of Quantification (LLOQ)
2.6.3. Accuracy, Precision, and Recovery
2.6.4. Stability and Matrix Effect
2.7. Application to a Pharmacokinetic Study
3. Results and Discussion
3.1. Method Development
3.1.1. Optimization of the HPLC-MS/MS Conditions
3.1.2. Optimization of Extraction Procedure
3.2. Method Validation
3.2.1. Specificity
3.2.2. Linearity and LLOQ
3.2.3. Precision, Accuracy
3.2.4. Extraction Recoveries and Matrix Effects
3.2.5. Stability
3.3. Pharmacokinetic Study
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, I.; Kim, H.; Moon, S.; Lee, H.; Kim, B. Overview of salvia miltiorrhiza as a potential therapeutic agent for various diseases: An update on efficacy and mechanisms of action. Antioxidants 2020, 9, 857. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Liu, J.; Zhao, X.; Zhang, Y.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2021, 11, 2189. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Liu, P. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Liu, Y.; Jiang, W.; Meng, X.; Zhang, W.; Chen, W.; Peng, D.; Xing, S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during Sweating processing. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Morris-Natschke, S.; Lee, K. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev. 2007, 27, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Chen, J.; Bi, D.; Gu, L.; Yao, L.; Li, X.; Li, H.; Xu, H.; Hu, Z.; Liu, Q.; et al. Specific degradation of endogenous Tau protein and inhibition of Tau fibrillation by tanshinone IIA through the ubiquitin-proteasome pathway. J. Agric. Food Chem. 2020, 68, 2054–2062. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Y.; Zhang, Y.; Zhang, M.; Sun, Y.; Liang, G.; Xu, J.; Zheng, J. Tanshinones inhibit hIAPP aggregation, disaggregate preformed hIAPP fibrils, and protect cultured cells. J. Mater. Chem. B 2017, 6, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, X.; Duan, Y.; Pan, X.; Sun, Y.; You, T.; Han, L.; Jin, Z.; Shang, W.; Yu, J.; et al. High-throughput screening identifies established drugs as SARS-CoV-2 PLpro inhibitors. Protein Cell 2021, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Du, F.; Jia, W.; Olaleye, O.; Xu, F.; Wang, F.; Li, L. Simultaneous determination of eight Danshen polyphenols in rat plasma and its application to a comparative pharmacokinetic study of DanHong injection and Danshen injection. J. Sep. Sci. 2017, 40, 1470–1481. [Google Scholar] [CrossRef]
- Liu, X.; Jin, M.; Zhang, M.; Li, T.; Sun, S.; Zhang, J.; Dai, J.; Wang, Y. The application of combined 1 H NMR-based metabolomics and transcriptomics techniques to explore phenolic acid biosynthesis in Salvia miltiorrhiza Bunge. J. Pharm. Biomed. Anal. 2019, 172, 126–138. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, Y.; Cai, R.; Guo, S.; Zhang, X.; Xue, Y.; Wang, J.; Huang, J.; Wang, J.; Gu, Y.; et al. Salvianolic acid A increases the accumulation of doxorubicin in brain tumors through Caveolae endocytosis: The regulatory mechanism of BTB permeability induced by Salvianolic acid A. Neuropharmacology 2020, 167, 107980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Yan, Z.; Xu, T.; Wu, X.; Pi, A.; Liu, Q.; Chai, H.; Li, S.; Dou, X. Inhibition of TLR4/MAPKs pathway contributes to the protection of salvianolic acid A against lipotoxicity-induced myocardial damage in cardiomyocytes and obese mice. Front. Pharmacol. 2021, 12, 76. [Google Scholar] [CrossRef]
- Chan, K.; Ho, W. Shing Anti-oxidative and hepatoprotective effects of lithospermic acid against carbon tetrachloride-induced liver oxidative damage in vitro and in vivo. Oncol. Rep. 2015, 34, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.; Rai, H.; Singh, G.; Singh, G.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, M.; Kenechukwu, N.; Gu, Y.; Chen, Y.; Zhong, S.; Gao, Y.; Cao, X.; Wang, L.; Liu, F.; et al. Rosmarinic acid ameliorates hypoxia/ischemia induced cognitive deficits and promotes remyelination. Neural Regen. Res. 2020, 15, 894–902. [Google Scholar] [CrossRef]
- Ma, W.; Peng, Y.; Wang, W.; Bian, Q.; Wang, N.; Lee, D.Y.W.; Dai, R. Pharmacokinetic comparison of five tanshinones in normal and arthritic rats after oral administration of Huo Luo Xiao Ling Dan or its single herb extract by UPLC-MS/MS. Biomed. Chromatogr. 2016, 30, 1573–1581. [Google Scholar] [CrossRef]
- Lu, P.; Xing, Y.; Xue, Z.; Ma, Z.; Zhang, B.; Peng, H.; Zhou, Q.; Liu, H.; Liu, Z.; Li, J. Pharmacokinetics of salvianolic acid B, rosmarinic acid and Danshensu in rat after pulmonary administration of Salvia miltiorrhiza polyphenolic acid solution. Biomed. Chromatogr. 2019, 33, e4561. [Google Scholar] [CrossRef]
- Xie, X.; Miao, J.; Sun, W.; Huang, J.; Li, D.; Li, S.; Tong, L.; Sun, G. Simultaneous determination and pharmacokinetic study of four phenolic acids in rat plasma using UFLC–MS/MS after intravenous administration of salvianolic acid for injection. J. Pharm. Biomed. Anal. 2017, 134, 53–59. [Google Scholar] [CrossRef]
- Lin, H.; Lin, T.; Chien, H.; Juang, Y.; Chen, C.; Wang, C.; Lai, C. A rapid, simple, and high-throughput UPLC-MS/MS method for simultaneous determination of bioactive constituents in Salvia miltiorrhiza with positive/negative ionization switching. J. Pharm. Biomed. Anal. 2018, 161, 94–100. [Google Scholar] [CrossRef]
- Su, T.; Yu, H.; Kwan, H.; Ma, X.; Cao, H.; Cheng, C.; Leung, A.; Chan, C.; Li, W.; Cao, H.; et al. Comparisons of the chemical profiles, cytotoxicities and anti-inflammatory effects of raw and rice wine-processed Herba Siegesbeckiae. J. Ethnopharmacol. 2014, 156, 365–369. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, H.; Li, Y.; Liu, Y.; Dai, W.; Fang, J.; Cao, C.; Die, Y.; Liu, Q.; Wang, C.; et al. Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 143, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, M.; Yi, Y.; Zeng, R.; Huang, Y.; Wu, P. Effects of Processed Radix Salviae Miltiorrhizae and Radix et Rhizoma Rhei with Wine on Functions of Blood Platelet and Anticoagulation of Rat. Chin. Tradit. Pat. Med. 2001, 23, 341–342. [Google Scholar] [CrossRef]
- Li, C.; Zhao, L.; Yang, Y.; Kang, W. Antimicrobial activity of Salvia miltiorrhiza and different processed products. Chin. Tradit. Pat. Med. 2011, 33, 1948–1951. [Google Scholar] [CrossRef]
- Cui, W.; Li, H.; Zhang, X.; Song, M.; Diao, J.; Zhang, D.; Wang, X. Analysis of five qualitative change compounds before and after wine processing of Salvia miltiorrhiza by UPLC-QE/MS. Chin. Tradit. Pat. Med. 2019, 41, 844–849. [Google Scholar] [CrossRef]
- FDA Guidance for Industry Bioanalytical Method Validation Guidance for Industry Bioanalytical Method Validation. 2018, 1–44. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry.
- Zhang, D.; Sun, L.; Li, H.; Cui, Y.; Liu, S.; Wu, P.; Zhao, D.; Zhao, P.; Zhang, X. Pharmacokinetic comparison of nine bioactive components in rat plasma following oral administration of raw and wine-processed Ligustri Lucidi Fructus by ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J. Sep. Sci. 2020, 43, 3995–4005. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Li, Q.; Feng, Y.; Dai, X.; Zhao, R.; Zhao, Y.; Jia, P.; Wang, S.; Yu, J.; Liao, S.; et al. Pharmacokinetics of 13 active components in a rat model of middle cerebral artery occlusion after intravenous injection of Radix Salviae miltiorrhizae-Lignum dalbergiae odoriferae prescription. J. Sep. Sci. 2020, 43, 531–546. [Google Scholar] [CrossRef]
- Hung, Y.; Wang, P.; Lin, T.; Yang, P.; You, J.; Pan, T. Functional redox proteomics reveal that salvia miltiorrhiza aqueous extract alleviates adriamycin-induced cardiomyopathy via inhibiting ROS-dependent apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 5136934. [Google Scholar] [CrossRef]
- Ning, Y.; Pei, K.; Cao, G.; Cai, H.; Liu, X.; Cao, L.; Zhang, S.; Cai, B. Comparative study on pharmacokinetics of four active compounds in rat plasma after oral administration of raw and wine processed chuanxiong rhizoma. Molecules 2020, 25, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Tan, S.; Zhang, Y.; Li, J.; Chai, C.; Li, J.; Cai, B. The effects of wine-processing on ascending and descending: The distribution of flavonoids in rat tissues after oral administration of crude and wine-processed Radix scutellariae. J. Ethnopharmacol. 2014, 155, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, B.; Li, Q.; He, J.; Wang, D.; Bi, K. Simultaneous determination of multiple active components in rat plasma using ultra-fast liquid chromatography with tandem mass spectrometry and application to a comparative pharmacokinetic study after oral administration of Suan-Zao-Ren decoction and Suan-Zao-Ren granule. J. Sep. Sci. 2017, 40, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, X.; Qiao, M.; Li, Y.; Cao, S.; Ding, L.; Feng, X.; Kang, N.; Zhang, D.; Qiu, F. Simultaneous determination of five isoflavones in rat plasma by LC-MS/MS: Comparative pharmacokinetic characteristics of Puerariae lobatae radix in normal and type 2 diabetic rats. J. Sep. Sci. 2019, 42, 2592–2601. [Google Scholar] [CrossRef] [PubMed]



| Analytes | ESI Mode | Precursor Ion (m/z) | Product Ion (m/z) | DP | CE |
|---|---|---|---|---|---|
| Tanshinone IIA | positive | 295.1 | 277.2 | 130 | 26 |
| Cryptotanshinone | positive | 297.1 | 279.1 | 100 | 29 |
| Dihydrotanshinone I | positive | 279.1 | 261.2 | 200 | 22 |
| Salvianolic acid A | negative | 493.3 | 295.1 | −100 | −24 |
| Rosmarinic acid | negative | 359.1 | 293.1 | −150 | −33 |
| Ferulic acid | negative | 193.1 | 134.1 | −100 | −26 |
| Lithospermic acid | negative | 537.1 | 493.1 | −68 | −11 |
| IS | positive | 256.2 | 167.1 | 200 | 16 |
| Analytes | Concentration Range (ng/mL) | Regression Equation | Correlation Coefficient (r) |
|---|---|---|---|
| Tanshinone IIA | 0.5−300 | Y = 0.828X + 0.0137 | 0.997 |
| Cryptotanshinone | 0.5−300 | Y = 0.125X + 0.0744 | 0.996 |
| Dihydrotanshinone I | 0.5−300 | Y = 0.0695X − 0.0205 | 0.997 |
| Salvianolic acid A | 0.5−300 | Y = 0.00478X + 0.0137 | 0.996 |
| Rosmarinic acid | 0.5−300 | Y = 0.00434X + 0.00434 | 0.998 |
| Ferulic acid | 0.5−300 | Y = 0.00332X + 0.00174 | 0.997 |
| Lithospermic acid | 0.5−300 | Y = 0.000721X + 0.00376 | 0.996 |
| Analytes | Spiked (ng/mL) | Intra-Day Precision RSD (%) | Inter-Day Precision RSD (%) | Intra-Day Accuracy RE (%) | Recovery Mean ± SD (%) | Matrix Effect Mean ± SD (%) |
|---|---|---|---|---|---|---|
| Tanshinone IIA | 0.5 | 6.2 | 7.4 | 6.4 | 87.4 ± 5.0 | 109.1 ± 3.4 |
| 5.0 | 5.2 | 5.8 | 4.3 | 89.4 ± 6.5 | 105.4 ± 4.2 | |
| 240 | 3.4 | 4.2 | 4.7 | 91.7 ± 4.2 | 110.3 ± 2.5 | |
| Cryptotanshinone | 0.5 | 7.8 | 8.9 | 2.7 | 85.3 ± 7.6 | 102.5 ± 1.7 |
| 5.0 | 5.9 | 4.2 | −1.5 | 89.2 ± 3.4 | 92.3 ± 2.5 | |
| 240 | 4.7 | 5.2 | 4.6 | 91.3 ± 6.1 | 97.4 ± 6.4 | |
| Dihydrotanshinone I | 0.5 | 8.1 | 6.7 | −2.1 | 89.4 ± 6.9 | 91.4 ± 7.1 |
| 5.0 | 3.5 | 5.5 | 6.2 | 91.2 ± 7.5 | 93.9 ± 2.0 | |
| 240 | 4.3 | 5.7 | 5.5 | 92.6 ± 8.4 | 98.1 ± 5.5 | |
| Salvianolic acid A | 0.5 | 7.2 | 9.3 | 3.2 | 106.7 ± 8.7 | 96.4 ± 4.1 |
| 5.0 | 6.8 | 5.7 | 0.8 | 87.9 ± 2.4 | 106.5 ± 3.4 | |
| 240 | 4.6 | 3.6 | 2.9 | 105.4 ± 4.7 | 103.7 ± 3.6 | |
| Rosmarinic acid | 0.5 | 4.1 | 5.2 | 4.5 | 94.6 ± 4.2 | 89.4 ± 5.7 |
| 5.0 | 2.4 | 2.2 | 1.4 | 92.7 ± 5.1 | 91.6 ± 6.8 | |
| 240 | 3.3 | 5.8 | 2.6 | 102.5 ± 7.8 | 95.5 ± 1.5 | |
| Ferulic acid | 0.5 | 9.2 | 8.7 | 5.5 | 87.3 ± 5.4 | 112.4 ± 2.4 |
| 5.0 | 6.5 | 6.7 | 6.2 | 91.6 ± 4.7 | 104.8 ± 5.1 | |
| 240 | 4.7 | 5.5 | 5.4 | 96.5 ± 6.4 | 105.4 ± 2.8 | |
| Lithospermic acid | 0.5 | 7.1 | 6.5 | 2.7 | 105.6 ± 8.7 | 92.4 ± 3.4 |
| 5.0 | 5.6 | 7.6 | 5.5 | 96.8 ± 7.9 | 89.7 ± 4.8 | |
| 240 | 2.8 | 4.0 | 6.2 | 90.9 ± 6.2 | 86.7 ± 6.5 | |
| IS | 400 | N.D. | N.D. | N.D. | 94.7 ± 5.4 | N.D. |
| Analytes | Spiked (ng/mL) | Stability at 4 °C for 24 h | Stability at −80 °C for 30 Days | Freeze-Thaw Stability | |||
|---|---|---|---|---|---|---|---|
| (RSD, %) | (RE, %) | (RSD, %) | (RE, %) | (RSD, %) | (RE, %) | ||
| Tanshinone IIA | 0.5 | 6.8 | 4.0 | 4.1 | 3.6 | 6.7 | 3.1 |
| 5.0 | 5.9 | −2.4 | 4.8 | 6.8 | 5.9 | −2.8 | |
| 240 | 6.5 | −2.6 | 1.6 | 2.0 | 3.0 | 4.8 | |
| Cryptotanshinone | 0.5 | 8.0 | −7.2 | 8.9 | 5.6 | 8.3 | 2.4 |
| 5.0 | 3.6 | 9.2 | 6.5 | 6.4 | 7.0 | 6.4 | |
| 240 | 3.2 | 2.4 | 5.9 | −3.7 | 2.8 | 1.9 | |
| Dihydrotanshinone I | 0.5 | 9.8 | −2.4 | 7.4 | −5.2 | 6.7 | −1.2 |
| 5.0 | 4.5 | 5.6 | 4.3 | 0.8 | 3.7 | 4.8 | |
| 240 | 1.9 | 1.9 | 4.0 | 3.2 | 3.0 | 4.3 | |
| Salvianolic acid A | 0.5 | 5.2 | 6.0 | 6.0 | 1.2 | 6.4 | 2.8 |
| 5.0 | 6.1 | 2.4 | 3.6 | 7.2 | 6.8 | −2.8 | |
| 240 | 1.7 | −0.9 | 4.8 | −3.6 | 4.6 | −3.3 | |
| Rosmarinic acid | 0.5 | 8.2 | −1.2 | 6.7 | −8.8 | 4.9 | −1.2 |
| 5.0 | 2.9 | 3.2 | 5.1 | 4.8 | 2.8 | 7.6 | |
| 240 | 1.3 | 2.5 | 3.6 | 4.1 | 3.0 | 4.3 | |
| Ferulic acid | 0.5 | 3.7 | −9.6 | 7.6 | 6.0 | 8.0 | 7.2 |
| 5.0 | 5.3 | 5.6 | 5.2 | 5.6 | 7.3 | 4.0 | |
| 240 | 3.2 | 3.6 | 6.2 | 1.5 | 4.4 | 3.0 | |
| Lithospermic acid | 0.5 | 7.2 | 4.8 | 3.5 | 1.6 | 4.0 | 8.4 |
| 5.0 | 3.5 | 8.4 | 4.2 | 9.2 | 8.6 | 3.6 | |
| 240 | 3.0 | 0.9 | 5.4 | 2.3 | 2.9 | 3.9 | |
| Analytes | Doses (mg/kg) | AUC0-t b (ng·h/mL) | AUC0-∞ (ng·h/mL) | Cmax (ng/mL) | CL/F (L/h/kg) | Tmax (h) | MRT (h) | T1/2 (h) | |
|---|---|---|---|---|---|---|---|---|---|
| CDS | Tanshinone IIA | 0.283 | 15.80 ± 5.62 | 15.80 ± 5.62 | 9.36 ± 3.17 * | 21.23 ± 9.60 | 0.58 ± 0.19 | 1.70 ± 0.29 | 1.73 ± 0.44 |
| Cryptotanshinone | 0.196 | 30.92 ± 11.95 | 30.97 ± 12.01 | 18.48 ± 5.91 | 7.36 ± 2.81 | 0.46 ± 0.09 | 1.97 ± 0.54 | 2.82 ± 1.56 | |
| Dihydrotanshinone I | 0.64 | 446.17 ± 94.44 * | 449.09 ± 92.53 * | 173.67 ± 31.12 * | 1.49 ± 0.28 * | 0.83 ± 0.24 | 2.73 ± 1.39 | 3.34 ± 3.58 | |
| Salvianolic acid A | 2.17 | 343.16 ± 57.06 | 354.79 ± 56.29 | 87.92 ± 13.82 | 6.50 ± 1.10 * | 0.50 ± 0.00 | 5.84 ± 0.93 | 9.12 ± 2.48 | |
| Rosmarinic acid | 5.05 | 57.63 ± 12.38 | 61.58 ± 16.73 | 31.88 ± 6.69 | 93.35 ± 27.27 | 0.25 ± 0.00 | 4.05 ± 3.05 | 6.65 ± 6.91 | |
| Ferulic acid | 0.149 | 3.41 ± 1.35 | 3.41 ± 1.35 | 4.14 ± 1.00 | 49.94 ± 17.00 | 0.58 ± 0.19 | 0.69 ± 0.23 | N.A. | |
| Lithospermic acid | 12.40 | 1093.00 ± 179.05 | 1116.94 ± 183.96 | 249.00 ± 35.22 | 11.65 ± 1.88 | 0.67 ± 0.24 | 6.89 ± 1.14 | 7.33 ± 1.74 | |
| WDS | Tanshinone IIA | 0.335 | 9.62 ± 2.85 | 9.60 ± 2.86 | 3.94 ± 1.12 * | 37.56 ± 9.28 | 0.67 ± 0.24 | 2.08 ± 0.80 | 2.06 ± 0.98 |
| Cryptotanshinone | 0.231 | 23.78 ± 8.35 | 24.54 ± 9.77 | 11.59 ± 3.63 | 10.95 ± 3.71 | 0.54 ± 0.22 | 2.51 ± 1.60 | 3.29 ± 3.47 | |
| Dihydrotanshinone I | 0.723 | 300.84 ± 78.25 * | 304.75 ± 79.61 * | 101.95 ± 15.35 * | 2.57 ± 0.70 * | 0.67 ± 0.24 | 2.84 ± 0.97 | 4.30 ± 4.81 | |
| Salvianolic acid A | 1.66 | 340.75 ± 65.30 | 364.27 ± 70.23 | 73.57 ± 14.13 | 17.27 ± 3.82 * | 0.58 ± 0.19 | 8.03 ± 3.17 | 10.26 ± 4.30 | |
| Rosmarinic acid | 5.64 | 47.58 ± 12.78 | 47.62 ± 12.79 | 27.32 ± 6.88 | 132.83 ± 55.11 | 0.33 ± 0.12 | 1.91 ± 0.71 | 1.98 ± 1.36 | |
| Ferulic acid | 0.165 | 3.73 ± 1.63 | 3.73 ± 1.63 | 4.28 ± 1.25 | 55.9 ± 28.50 | 0.40 ± 0.12 | 0.62 ± 0.16 | N.A. | |
| Lithospermic acid | 10.1 | 1058.43 ± 256.90 | 1131.25 ± 227.92 | 238.67 ± 49.66 | 10.16 ± 2.58 | 0.58 ± 0.19 | 7.19 ± 1.61 | 10.51 ± 5.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cui, W.; Liu, X.; Wang, N.; Kong, W.; Sui, J.; Li, H.; Wang, S. Simultaneous Determination of Seven Bioactive Constituents from Salvia miltiorrhiza in Rat Plasma by HPLC-MS/MS: Application to a Comparative Pharmacokinetic Study. Separations 2021, 8, 93. https://doi.org/10.3390/separations8070093
Zhang Y, Cui W, Liu X, Wang N, Kong W, Sui J, Li H, Wang S. Simultaneous Determination of Seven Bioactive Constituents from Salvia miltiorrhiza in Rat Plasma by HPLC-MS/MS: Application to a Comparative Pharmacokinetic Study. Separations. 2021; 8(7):93. https://doi.org/10.3390/separations8070093
Chicago/Turabian StyleZhang, Yanli, Weiliang Cui, Xianghong Liu, Ning Wang, Wenru Kong, Junyu Sui, Huifen Li, and Shuqi Wang. 2021. "Simultaneous Determination of Seven Bioactive Constituents from Salvia miltiorrhiza in Rat Plasma by HPLC-MS/MS: Application to a Comparative Pharmacokinetic Study" Separations 8, no. 7: 93. https://doi.org/10.3390/separations8070093
APA StyleZhang, Y., Cui, W., Liu, X., Wang, N., Kong, W., Sui, J., Li, H., & Wang, S. (2021). Simultaneous Determination of Seven Bioactive Constituents from Salvia miltiorrhiza in Rat Plasma by HPLC-MS/MS: Application to a Comparative Pharmacokinetic Study. Separations, 8(7), 93. https://doi.org/10.3390/separations8070093