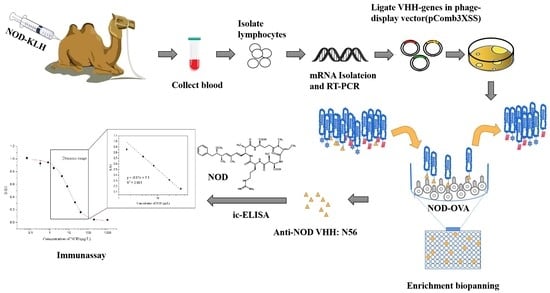
Preparation of Specific Nanobodies and Their Application in the Rapid Detection of Nodularin-R in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Construction of a Phage-Displayed Nanobody Library
2.4. Biopanning and Identification of Nanobody Clones for NOD-R
2.5. Expression and Characteristics of Nanobodies
2.6. Establishment of an Indirect Competitive ELISA Based on the Nanobody N56
2.7. Analysis of Water Samples by Ic-ELISA for NOD-R Detection
3. Results and Discussion
3.1. Selection of Anti-NOD-R Nanobodies from the Phage Display Library
3.2. Preparation and Characterization Analysis of the Nanobody N56
3.3. Establishment of an Ic-ELISA Based on the Nanobody N56
3.4. Water Sample Analysis by ELISA and UPLC–MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Wang, C.C.; Petty, E.E.; Smith, C.M. Rapid and Efficient Analysis of Microcystins, Nodularin, Cylindrospermopsin, and Anatoxin-a in Drinking Water by LC Tandem MS. J. AOAC Int. 2016, 99, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, L.; Wang, M.; Hu, T. Comprehensive insights into the occurrence and toxicological issues of nodularins. Mar. Pollut. Bull. 2020, 162, 111884. [Google Scholar] [CrossRef]
- Ohta, T.; Sueoka, E.; Iida, N.; Komori, A.; Suganuma, M.; Nishiwaki, R.; Tatematsu, M.; Kim, S.J.; Carmichael, W.W.; Fujiki, H. Nodularin, a potent inhibitor of protein phosphatases 1 and 2A, is a new environmental carcinogen in male F344 rat liver. Cancer Res. 1994, 54, 6402–6406. [Google Scholar]
- Zhou, Z.; Feng, D.; Liang, W.; Lu, X.; Shi, X.; Zhao, C.; Xu, G. Synthesis of metal-organic framework-5@chitosan material for the analysis of microcystins and nodularin based on ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1623, 461198. [Google Scholar] [CrossRef] [PubMed]
- Toruńska-Sitarz, A.; Kotlarska, E.; Mazur-Marzec, H. Biodegradation of nodularin and other nonribosomal peptides by the Baltic bacteria. Int. Biodeterior. Biodegrad. 2018, 134, 48–57. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L.; Miller, T. Variable Cyanobacterial Toxin and Metabolite Profiles across Six Eutrophic Lakes of Differing Physiochemical Characteristics. Toxins 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Cyanobacterial toxins: Microcystin-LR. In Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Fitzgerald, D.J.; Cunliffe, D.A.; Burch, M.D. Development of health alerts for cyanobacteria and related toxins in drinking water in South Australia. Environ Toxicol. Environ. Toxicol. Int. J. 1999, 14, 203–209. [Google Scholar] [CrossRef]
- Jussi, M. Chromatography of microcystins. Anal. Chim. Acta 1997, 352, 277–298. [Google Scholar]
- Marcel, E.; Hans, V.D.; Peter, J. Rapid typing and elucidation of new secondary metabolites of intact cyanobacteria using MALDI-TOF mass spectrometry. Nat. Biotechnol. 1997, 15, 906–909. [Google Scholar]
- Meriluoto, J.; Karlsson, K.; Spoof, L. High-Throughput Screening of Ten Microcystins and Nodularins, Cyanobacterial Peptide Hepatotoxins, by Reversed-Phase Liquid Chromatography-Electrospray Ionisation Mass Spectrometry. Chromatographia 2004, 59, 291–298. [Google Scholar]
- Ouyang, S.; Hu, B.; Zhou, R.; Liu, D.; Peng, D.; Li, Z.; Li, Z.; Jiao, B.; Wang, L. Rapid and sensitive detection of nodularin-R in water by a label-free BLI aptasensor. Analyst 2018, 143, 4316–4322. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Samdal, I.A.; Ballot, A.; Løvberg, K.E.; Miles, C.O. Multihapten Approach Leading to a Sensitive ELISA with Broad Cross-Reactivity to Microcystins and Nodularin. Environ. Sci. Technol. 2014, 48, 8035–8043. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, Y.S.; Zhi, B.H.; Lu, S.Y.; Ren, H.L.; Zhang, Y.Y.; Li, Z.H.; Shen, Q.F.; Meng, X.M.; Liu, Z.S.; et al. Detection of nodularin based on a monoclonal antibody in water and aquatic fish samples. Food Control 2011, 22, 797–800. [Google Scholar] [CrossRef]
- Lu, N.; Ling, L.; Guan, T.; Wang, L.; Wang, D.; Zhou, J.; Ruan, T.; Shen, X.; Li, X.; Sun, Y.; et al. Broad-specificity ELISA with a heterogeneous strategy for sensitive detection of microcystins and nodularin. Toxicon 2019, 175, 44–48. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N. Naturally occurring antibodies devoid of light chains. Nat. Cell Biol. 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Kim, H.J.; McCoy, M.R.; Majkova, Z.; Dechant, J.E.; Gee, S.J.; Tabares-da Rosa, S.; González-Sapienza, G.G.; Hammock, B.D. Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Anal. Chem. 2012, 84, 1165–1171. [Google Scholar] [CrossRef] [Green Version]
- Bever, C.S.; Dong, J.X.; Vasylieva, N.; Barnych, B.; Cui, Y.; Xu, Z.L.; Hammock, B.D.; Gee, S.J. VHH antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016, 408, 5985–6002. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, Z.; Liu, X.; Deng, X.; Tang, Z. Development of a Nanobody-Based Competitive Dot ELISA for Visual Screening of Ochratoxin A in Cereals. Food Anal. Methods 2017, 10, 3558–3564. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, Z.L.; Wang, F.; Cai, J.; Dong, J.X.; Zhang, J.R.; Si, R.; Wang, C.L.; Wang, Y.; Shen, Y.D.; et al. Isolation of Bactrian camel Single Domain Antibody for Parathion and Development of One-Step dc-FEIA Method Using VHH-Alkaline Phosphatase Fusion Protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef]
- Ren, W.; Li, Z.; Xu, Y.; Wan, D.; Barnych, B.; Li, Y.; Tu, Z.; He, Q.; Fu, J.; Hammock, B.D. One-Step Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Fusion for Detection of Aflatoxin B1 in Cereal. J. Agric. Food Chem. 2019, 67, 5221–5229. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Z.; Ding, G.; Li, J.; Vasylieva, N.; Li, Q.X.; Li, D.; Gee, S.J.; Hammock, B.D.; Xu, T. Development of a one-step immunoassay for triazophos using camel single-domain antibody–alkaline phosphatase fusion protein. Anal. Bioanal. Chem. 2019, 411, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Wang, Y.; Dong, J.X.; Yang, J.Y.; Zhang, Y.Q.; Wang, F.; Si, R.; Xu, Z.L.; Wang, H.; Xiao, Z.L.; et al. Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules 2019, 9, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.Y.; Wang, Y.; Zhang, Y.F.; Wang, F.; Liang, Y.F.; Yang, J.Y.; Xu, Z.L.; Shen, Y.D.; Wang, H. Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine. Biomolecules 2021, 11, 167. [Google Scholar] [CrossRef]
- De Meyer, T.; Muyldermans, S.; Depicker, A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 32, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bever, C.R.S.; Majkova, Z.; Dechant, J.E.; Yang, J.; Gee, S.J.; Xu, T.; Hammock, B.D. Heterologous Antigen Selection of Camelid Heavy Chain Single Domain Antibodies against Tetrabromobisphenol A. Anal. Chem. 2014, 86, 8296–8302. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, Z.F.; Yang, Y.Y.; Wan, D.B.; Vasylieva, N.; Zhang, Y.Q.; Cai, J.; Wang, H.; Shen, Y.D.; Xu, Z.L.; et al. Chemiluminescent Enzyme Immunoassay and Bioluminescent Enzyme Immunoassay for Tenuazonic Acid Mycotoxin by Exploitation of Nanobody and Nanobody-Nanoluciferase Fusion. Anal. Chem. 2020, 92, 11935–11942. [Google Scholar] [CrossRef]
- Zabetakis, D.; Olson, M.A.; Anderson, G.; Legler, P.M.; Goldman, E.R. Evaluation of Disulfide Bond Position to Enhance the Thermal Stability of a Highly Stable Single Domain Antibody. PLoS ONE 2014, 9, e115405. [Google Scholar] [CrossRef]
- Pírez-Schirmer, M.; Rossotti, M.; Badagian, N.; Leizagoyen, C.; Brena, B.M.; Gonzalez-Sapienza, G. Comparison of Three Antihapten VHH Selection Strategies for the Development of Highly Sensitive Immunoassays for Microcystins. Anal. Chem. 2017, 89, 6800–6806. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Liu, L.; Li, J.; Liu, X.; Zhang, X.; Liu, Y.; Zhang, C.; Liu, X. Microcystin-LR nanobody screening from an alpaca phage display nanobody library and its expression and application. Ecotoxicol. Environ. Saf. 2018, 151, 220–227. [Google Scholar] [CrossRef]








| Primer Names | Nucleotide Sequences (5′→3′) |
|---|---|
| CALL001 | GTCCTGGCTGCTCTTCTACAAGG |
| CALL002 | GGTACGTGCTGTTGAACTGTTCC |
| Fr4-SfiI | ACTGGCCCAGGCGGCCGAGGTGCAGCTGSWGSAKTCKG |
| Fr1-SfiI | ACTGGCCGGCCTGGCCTGAGGAGACGGTGACCWGGGTC |
| Algal Toxins | Structure | IC50(µg/L) | CRs |
|---|---|---|---|
| NOD |  | 9.94 | 100.0% |
| MC-LR |  | 8.24 | 120.6% |
| MC-LA |  | 42.41 | 23.4% |
| MC-LY |  | 60.78 | 16.4% |
| MC-LW |  | 180.81 | 5.5% |
| MC-LF |  | 113.79 | 8.7% |
| MC-YR |  | 7.85 | 126.6% |
| MC-WR |  | 13.50 | 73.6% |
| MC-RR |  | 11.87 | 83.7% |
| Ic-ELISA | UPLC-MS/MS | R2 | |||||
|---|---|---|---|---|---|---|---|
| Spiked Level (µg/L) | Found ± SD (µg/L) | Recovery (%) | CV (%) | Found ± SD (µg/L) | Recovery (%) | CV (%) | |
| 0 | / | / | / | / | / | / | 0.999 |
| 0.5 | 0.59 ± 0.02 | 117.7 | 3.6 | 0.53 ± 0.01 | 106.0 | 1.9 | |
| 1 | 1.11 ± 0.07 | 111.0 | 5.8 | 1.07 ± 0.02 | 107.0 | 1.9 | |
| 2 | 2.39 ± 0.02 | 119.5 | 2.0 | 2.33 ± 0.04 | 116.5 | 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Si, R.; Wu, G.; Wang, Y.; Fang, R.; Liu, F.; Wang, F.; Lei, H.; Shen, Y.; Zhang, Q.; et al. Preparation of Specific Nanobodies and Their Application in the Rapid Detection of Nodularin-R in Water Samples. Foods 2021, 10, 2758. https://doi.org/10.3390/foods10112758
Yang J, Si R, Wu G, Wang Y, Fang R, Liu F, Wang F, Lei H, Shen Y, Zhang Q, et al. Preparation of Specific Nanobodies and Their Application in the Rapid Detection of Nodularin-R in Water Samples. Foods. 2021; 10(11):2758. https://doi.org/10.3390/foods10112758
Chicago/Turabian StyleYang, Jinyi, Rui Si, Guangpei Wu, Yu Wang, Ruyu Fang, Fei Liu, Feng Wang, Hongtao Lei, Yudong Shen, Qi Zhang, and et al. 2021. "Preparation of Specific Nanobodies and Their Application in the Rapid Detection of Nodularin-R in Water Samples" Foods 10, no. 11: 2758. https://doi.org/10.3390/foods10112758
APA StyleYang, J., Si, R., Wu, G., Wang, Y., Fang, R., Liu, F., Wang, F., Lei, H., Shen, Y., Zhang, Q., & Wang, H. (2021). Preparation of Specific Nanobodies and Their Application in the Rapid Detection of Nodularin-R in Water Samples. Foods, 10(11), 2758. https://doi.org/10.3390/foods10112758