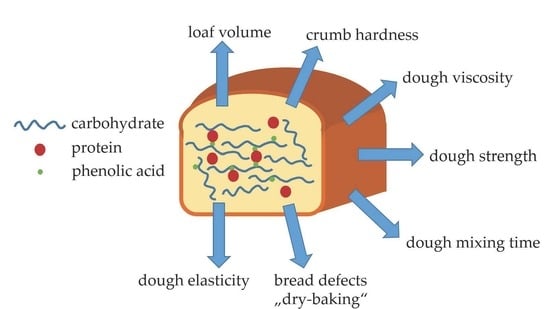
Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties
Abstract
:1. Introduction
2. Phenolic Acids
3. Interactions of PAs with Proteins
4. Interactions of PAs with Carbohydrates
5. Influence of Interactions between PAs and Proteins on Dough and Bread Properties
6. Influence of Interactions between PAs and Carbohydrates, Especially Starch and Pentosans, on Dough and Bread Properties
7. (Ternary) Interactions between PAs, Proteins, and Carbohydrates, and Their Influence on Dough and Bread Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekara, A. Phenolic Acids; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-814045-1. [Google Scholar]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, D.E.; Hurst, R.D. Review Polyphenolic phytochemicals—Just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.S.M. The Impact of Milling and Thermal Processing on Phenolic Compounds in Cereal Grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef]
- Rubino, M.I.; Arntfield, S.D.; Nadon, C.A.; Bernatsky, A. Phenolic protein interactions in relation to the gelation properties of canola protein. Food Res. Int. 1996, 29, 653–659. [Google Scholar] [CrossRef]
- Sastry, S.; Rao, N. Binding of Chlorogenic Acid by the Isolated Polyphenol-Free 11s Protein of Sunflower (Helianthus annuus) Seed. J. Agric. 1990, 38, 2103–2110. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; Van Koningsveld, G.A.; De Jong, G.A.H.; Voragen, A.G.J. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef] [PubMed]
- Trnkova, L.; Bousova, I.; Kubicek, V.; Drsata, J.; Trnková, L.; Bousová, I.; Kubìcek, V.; Drsata, J. Binding of naturally occurring hydroxycinnamic acids to bovine serum albumin. Nat. Sci. 2010, 2, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Nagy, K.; Courtet-Compondu, M.C.; Williamson, G.; Rezzi, S.; Kussmann, M.; Rytz, A. Non-covalent binding of proteins to polyphenols correlates with their amino acid sequence. Food Chem. 2012, 132, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Swieca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic-protein interactions: Effects on food properties and health benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kroll, J.; Hohl, U.C. Model studies on reactions of plant phenols with whey proteins. Nahr. Food 2001, 45, 72–81. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S. Nature of hydroxycinnamate-protein interactions. Phytochem. Rev. 2010, 9, 93–109. [Google Scholar] [CrossRef]
- Pierpoint, W.S. o-Quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem. J. 1969, 112, 609–616. [Google Scholar] [CrossRef]
- Pierpoint, W.S. o-Quinones formed in plant extracts. Their reaction with bovine serum albumin. Biochem. J. 1969, 112, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Rawel, H.M.; Pietruschinski, N.; Kroll, J. In vitro inhibition of α-chymotryptic activity by phenolic compounds. J. Sci. Food Agric. 2001, 81, 1512–1521. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kruse, H.-P.; Kroll, J. Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem. 2002, 78, 443–455. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Ferraro, V.; Madureira, A.R.; Sarmento, B.; Gomes, A.; Pintado, M.E. Study of the interactions between rosmarinic acid and bovine milk whey protein α-Lactalbumin, β-Lactoglobulin and Lactoferrin. Food Res. Int. 2015, 77, 450–459. [Google Scholar] [CrossRef]
- Shen, F.; Niu, F.; Li, J.; Su, Y.; Liu, Y.; Yang, Y. Interactions between tea polyphenol and two kinds of typical egg white proteins-ovalbumin and lysozyme: Effect on the gastrointestinal digestion of both proteins in vitro. Food Res. Int. 2014, 59, 100–107. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Proposed mechanism for the effect of polyphenols on the heat stability of milk. Int. Dairy J. 1999, 9, 523–536. [Google Scholar] [CrossRef]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Effect of phenolic compounds on the properties of porcine plasma protein-based film. Food Hydrocoll. 2009, 23, 736–741. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X.; Yi, J. Physicochemical properties of gelatin gels from walleye pollock (Theragra chalcogramma) skin cross-linked by gallic acid and rutin. Food Hydrocoll. 2011, 25, 907–914. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Xu, H.; Zhang, T.; Lin, X.; Wu, X. Effect of covalent interaction with chlorogenic acid on the allergenic capacity of ovalbumin. J. Agric. Food Chem. 2018, 66, 9794–9800. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Covalent modification of flaxseed protein isolate by phenolic compounds and the structure and functional properties of the adducts. Food Chem. 2019, 293, 463–471. [Google Scholar] [CrossRef]
- Ojha, H.; Mishra, K.; Hassan, M.I.; Chaudhury, N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta 2012, 548, 56–64. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Liu, M.; Liu, Z.; Xu, H.; Lai, F. Analysis of binding interaction between (-)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 164–168. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Corke, H. Effect of phenolic compounds on the pasting and textural properties of wheat starch. Starch/Staerke 2008, 60, 609–616. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Corke, H. Effect of phytochemical extracts on the pasting, thermal, and gelling properties of wheat starch. Food Chem. 2009, 112, 919–923. [Google Scholar] [CrossRef]
- Van Hung, P.; Phat, N.H.; Phi, N.T.L. Physicochemical properties and antioxidant capacity of debranched starch-ferulic acid complexes. Starch/Staerke 2013, 65, 382–389. [Google Scholar] [CrossRef]
- Kandil, A.; Li, J.; Vasanthan, T.; Bressler, D.C. Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. J. Agric. Food Chem. 2012, 60, 8444–8449. [Google Scholar] [CrossRef]
- Bravo-Núñez, Á.; Garzón, R.; Rosell, C.M.; Gómez, M. Evaluation of Starch—Protein Interactions as a Function of pH. Foods 2019, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Buksa, K. Application of model bread baking in the examination of arabinoxylan—Protein complexes in rye bread. Carbohydr. Polym. 2016, 148, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Buksa, K.; Krystyjan, M. Arabinoxylan–starch–protein interactions in specially modified rye dough during a simulated baking process. Food Chem. 2019, 287, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Boskov Hansen, H.; Andreasen, M.F.; Nielsen, M.M.; Larsen, L.M.; Bach Knudsen, K.E.; Meyer, A.S.; Christensen, L.P.; Hansen, A. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of Plant Phenolics with Food Proteins and Enzymes under Special Consideration of Covalent Bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Ebermann, R.; Elmadfa, I. Phenolische Verbindungen als Bestandteile von Lebensmitteln. In Lehrbuch Lebensmittelchemie und Ernährung; Springer: Berlin/Heidelberg, Germany, 2011; pp. 191–214. [Google Scholar] [CrossRef]
- Kayser, O.; Averesch, N. Technische Biochemie: Die Biochemie und Industrielle Nutzung von Naturstoffen; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 3658055480. [Google Scholar]
- Singleton, V.L.; Cilliers, J.J.L. Characterization of the Products of Nonenzymic Autoxidative Phenolic Reactions in a Caffeic Acid Model System. J. Agric. Food Chem. 1991, 39, 1298–1303. [Google Scholar] [CrossRef]
- Selinheimo, E.; Autio, K.; Kruus, K.; Buchert, J. Elucidating the mechanism of laccase and tyrosinase in wheat bread making. J. Agric. Food Chem. 2007, 55, 6357–6365. [Google Scholar] [CrossRef] [PubMed]
- Selinheimo, E.; Lampila, P.; Mattinen, M.-L.; Buchert, J. Formation of Protein—Oligosaccharide Conjugates by Laccase and Tyrosinase. J. Agric. Food Chem. 2008, 56, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S. Possibilities and limitations in the analysis of covalent interactions between phenolic compounds and proteins. Food Res. Int. 2014, 65, 13–19. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Funk, C.; Steinhart, H. Structural elucidation of new ferulic acid-containing phenolic dimers and trimers isolated from maize bran. Tetrahedron Lett. 2005, 46, 5845–5850. [Google Scholar] [CrossRef]
- Bunzel, M.; Steinhart, H. Strukturmerkmale von ballaststoffkomponenten. Chem. Unserer Zeit 2003, 37, 188–196. [Google Scholar] [CrossRef]
- Jilek, M.L.; Bunzel, M. Dehydrotriferulic and dehydrodiferulic acid profiles of cereal and pseudocereal flours. Cereal Chem. 2013, 90, 507–514. [Google Scholar] [CrossRef]
- Renger, A.; Steinhart, H. Ferulic acid dehydrodimers as structural elements in cereal dietary fibre. Eur. Food Res. Technol. 2000, 211, 422–428. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Milenković, D.; Stepanić, V. Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.A. Interactions of Food Proteins with Plant Phenolics-Modulation of Structural, Techno- and Bio-Functional Properties of Proteins; University of Potsdam: Potsdam, Germany, 2013. [Google Scholar]
- Prigent, S.V.E. Interactions of Phenolic Compounds with Globular Proteins and Their Effects on Food-Related Functional Properties; Wageningen University: Wageningen, The Netherlands, 2005. [Google Scholar]
- Theertha Prasad, D. Studies on the Interaction of Sunflower Albumins with Chlorogenic Acid. J. Agric. Food Chem. 1988, 36, 450–452. [Google Scholar] [CrossRef]
- Sabir, M.A.; Sosulski, F.W.; Finlayson, A.J. Chlorogenic Acid-Protein Interactions in Sunflower. J. Agric. Food Chem. 1974, 22, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.D.; Battaile, J. Plant phenolic compounds and the isolation of plant enzymes. Phytochemistry 1966, 5, 423–438. [Google Scholar] [CrossRef]
- Murray, N.J.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. Eur. J. Biochem. 1994, 219, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Frey, S.K.; Meidtner, K.; Kroll, J.; Schweigert, F.J. Determining the binding affinities of phenolic compounds to proteins by quenching of the intrinsic tryptophan fluorescence. Mol. Nutr. Food Res. 2006, 50, 705–713. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.; Zhong, M.; Guo, J.; Wang, W.Z.; Zhu, R. Comparative studies on the interaction of caffeic acid, chlorogenic acid and ferulic acid with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 680–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Du, M.; Zhou, F. Comparative studies of the interaction between ferulic acid and bovine serum albumin by ACE and surface plasmon resonance. Electrophoresis 2007, 28, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liang, Q.; Luo, T.; Wang, Y.; Luo, G. Study on interactions of phenolic acid-like drug candidates with bovine serum albumin by capillary electrophoresis and fluorescence spectroscopy. J. Solution Chem. 2010, 39, 1653–1664. [Google Scholar] [CrossRef]
- Takeda, K.; Wada, A.; Yamamoto, K.; Moriyama, Y.; Aoki, K. Conformational change of bovine serum albumin by heat treatment. J. Protein Chem. 1989, 8, 653–659. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and qualitative evaluation of adsorption/desorption of bovine serum albumin on hydrophilic and hydrophobic surfaces. Langmuir 2009, 25, 11614–11620. [Google Scholar] [CrossRef]
- Abdollahi, K.; Ince, C.; Condict, L.; Hung, A.; Kasapis, S. Combined spectroscopic and molecular docking study on the pH dependence of molecular interactions between β-lactoglobulin and ferulic acid. Food Hydrocoll. 2020, 101, 105461. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Xie, M.X.; Li, S.; Jiang, M.; Wang, Y.D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta-Gen. Subj. 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sheng, Y.N.; Feng, Y.C.; Diao, J.J.; Wang, C.Y.; Zhang, D.J. Changes in structural and functional properties of globulin-polyphenol complexes in mung beans: Exploration under different interaction ratios and heat treatment conditions. Int. J. Food Sci. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Ali, M.; Keppler, J.K.; Coenye, T.; Schwarz, K. Covalent Whey Protein–Rosmarinic Acid Interactions: A Comparison of Alkaline and Enzymatic Modifications on Physicochemical, Antioxidative, and Antibacterial Properties. J. Food Sci. 2018, 83, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Prigent, S.V.E.; Voragen, A.G.J.; Visser, A.J.W.G.; Van Koningsveld, G.A.; Gruppen, H. Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2007, 87, 2502–2510. [Google Scholar] [CrossRef]
- Namiki, M.; Yabuta, G.; Koizumi, Y.; Yano, M. Development of free radical products during the greening reaction of caffeic acid esters (or chlorogenic acid) and a primary amino compound. Biosci. Biotechnol. Biochem. 2001, 65, 2131–2136. [Google Scholar] [CrossRef] [Green Version]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A New Look on Protein-Polyphenol Complexation during Honey Storage: Is This a Random or Organized Event with the Help of Dirigent-Like Proteins? PLoS ONE 2013, 8, e72897. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Ou, S.; Wang, Y.; Tang, S.; Huang, C.; Jackson, M.G. Role of ferulic acid in preparing edible films from soy protein isolate. J. Food Eng. 2005, 70, 205–210. [Google Scholar] [CrossRef]
- Junwen, L.; Tiejing, L.; Xinhuai, Z. Hydrogen peroxide and ferulic acid-mediated oxidative cross-linking of casein catalyzed by horseradish peroxidase and the impacts on emulsifying property and microstructure of acidified gel. Afr. J. Biotechnol. 2009, 8, 6993–6999. [Google Scholar] [CrossRef]
- Tantoush, Z.; Stanic, D.; Stojadinovic, M.; Ognjenovic, J.; Mihajlovic, L.; Atanaskovic-Markovic, M.; Cirkovic Velickovic, T. Digestibility and allergenicity of β-lactoglobulin following laccase-mediated cross-linking in the presence of sour cherry phenolics. Food Chem. 2011, 125, 84–91. [Google Scholar] [CrossRef]
- Elsayed, S.; Stavseng, L. Epitope mapping of region 11-70 of ovalbumin (Gald I) using five synthetic peptides. Int. Arch. Allergy Immunol. 1994, 104, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; She, C.H. Significance of phenol-protein interactions in modifying the antioxidant capacity of peas. J. Agric. Food Chem. 2006, 54, 8491–8494. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B. Wheat bread enriched with green coffee—In vitro bioaccessibility and bioavailability of phenolics and antioxidant activity. Food Chem. 2017, 221, 1451–1457. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A. Noncovalent Interactions of Sea Buckthorn Polyphenols with Casein and Whey Proteins: Effect on the Stability, Antioxidant Potential, and Bioaccessibility of Polyphenols. ACS Food Sci. Technol. 2021, 1, 1206–1214. [Google Scholar] [CrossRef]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef]
- Chang, K.; Liu, J.; Jiang, W.; Zhang, R.; Zhang, T.; Liu, B. Ferulic acid-ovalbumin protein nanoparticles: Structure and foaming behavior. Food Res. Int. 2020, 136, 109311. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L.; Wang, Z. Digestibility and supramolecular structural changes of maize starch by non-covalent interactions with gallic acid. Food Funct. 2017, 8, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pernell, C.; Ferruzzi, M.G. Complexation with phenolic acids affect rheological properties and digestibility of potato starch and maize amylopectin. Food Hydrocoll. 2018, 77, 843–852. [Google Scholar] [CrossRef]
- Li, M.; Ndiaye, C.; Corbin, S.; Foegeding, E.A.; Ferruzzi, M.G. Starch-phenolic complexes are built on physical CH-π interactions and can persist after hydrothermal treatments altering hydrodynamic radius and digestibility of model starch-based foods. Food Chem. 2020, 308, 125577. [Google Scholar] [CrossRef]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, L.; Thibault, J.F. Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans. J. Sci. Food Agric. 1999, 79, 396–402. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Physico-chemical characterization of starch ferulates of different degrees of substitution. Food Chem. 2007, 105, 579–589. [Google Scholar] [CrossRef]
- Ou, S.; Yang, A.L.A. A study on synthesis of starch ferulate and its biological properties. Food Chem. 2001, 74, 91–95. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T.; Fulcher, R.G. Ferulic acid fluorescence intensity profiles and concentration measured by HPLC in pigmented and non-pigmented cereals. Food Res. Int. 2013, 52, 109–118. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Johnson, K.G.; Silva, M.C.; Mackenzie, C.R.; Schneider, H.; Fontana, J.D. Microbial degradation of hemicellulosic materials. Appl. Biochem. Biotechnol. 1989, 20–21, 245–258. [Google Scholar] [CrossRef]
- Piot, O.; Autran, J.C.; Manfait, M. Spatial distribution of protein and phenolic constituents in wheat grain as probed by confocal raman microspectroscopy. J. Cereal Sci. 2000, 32, 57–71. [Google Scholar] [CrossRef]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of phenolic acids in barley by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Stone, B.A. Studies on wheat endosperm. Aust. J. Biol. Sci. 1973, 26, 793–812. [Google Scholar]
- Iiyama, K.; Lam, T.B.T.; Stone, B.A. Covalent cross-links in the cell wall. Plant Physiol. 1994, 104, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrewartha, K.A.; Phillips, D.R.; Stone, B.A. Solution properties of wheat-flour arabinoxylans and enzymically modified arabinoxylans. Carbohydr. Res. 1979, 77, 191–204. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Guerrero, V.; Salas-Muñoz, E. Feruloylated arabinoxylans and arabinoxylan gels: Structure, sources and applications. Phytochem. Rev. 2010, 9, 111–120. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Brüning, P.; Steinhart, H. Structural identification of dehydrotriferulic and dehydrotetraferulic acids isolated from insoluble maize bran fiber. J. Agric. Food Chem. 2006, 54, 6409–6418. [Google Scholar] [CrossRef]
- Dobberstein, D.; Bunzel, M. Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. J. Agric. Food Chem. 2010, 58, 8927–8935. [Google Scholar] [CrossRef]
- Harris, P.J.; Trethewey, J.A.K. The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochem. Rev. 2010, 9, 19–33. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biochim. Biophys. Acta-Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kong, L.; Zhao, Y.; Tan, L.; Zhang, J.; Du, Z.; Zhang, H. Lipophilization and molecular encapsulation of p-coumaric acid by amylose inclusion complex. Food Hydrocoll. 2019, 93, 270–275. [Google Scholar] [CrossRef]
- Obiro, W.C.C.; Sinha Ray, S.; Emmambux, M.N.N. V-amylose Structural Characteristics, Methods of Preparation, Significance, and Potential Applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef] [Green Version]
- Immel, S.; Lichtenthaler, F.W. The Hydrophobic Topographies of Amylose and its Blue Iodine Complex[1,2]. Starch/Staerke 2000, 52, 1–8. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Molecular Encapsulation of Ascorbyl Palmitate in Preformed V-Type Starch and Amylose; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 111, ISBN 1814863613. [Google Scholar]
- Uchino, T.; Tozuka, Y.; Oguchi, T.; Yamamoto, K. The change of the structure of amylose during the inclusion of 2-Naphthol in sealed-heating process. J. Incl. Phenom. 2001, 39, 145–149. [Google Scholar] [CrossRef]
- Le Bail, P.; Chauvet, B.; Simonin, H.; Rondeau-Mouro, C.; Pontoire, B.; De Carvalho, M.; Le-Bail, A. Formation and stability of amylose ligand complexes formed by high pressure treatment. Innov. Food Sci. Emerg. Technol. 2013, 18, 1–6. [Google Scholar] [CrossRef]
- Le-Bail, P.; Lorentz, C.; Pencreac’h, G.; Soultani-Vigneron, S.; Pontoire, B.; Giraldo, L.J.L.; Villeneuve, P.; Hendrickx, J.; Tran, V. Trapping by amylose of the aliphatic chain grafted onto chlorogenic acid: Importance of the graft position. Carbohydr. Polym. 2015, 117, 910–916. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Godet, M.C.; Tran, V.; Colonna, P.; Buleon, A.; Pezolet, M. Inclusion/exclusion of fatty acids in amylose complexes as a function of the fatty acid chain length. Int. J. Biol. Macromol. 1995, 17, 405–408. [Google Scholar] [CrossRef]
- Godet, M.C.; Buléon, A.; Tran, V.; Colonna, P. Structural features of fatty acid-amylose complexes. Carbohydr. Polym. 1993, 21, 91–95. [Google Scholar] [CrossRef]
- Rappenecker, G.; Zugenmaier, P. Detailed refinement of the crystal structure of Vh-amylose. Carbohydr. Res. 1981, 89, 11–19. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Yi, J.; Liang, J.; Liu, Y.; Zhang, L.M. Preparation, characterization, and properties of amylose-ibuprofen inclusion complexes. Starch/Staerke 2013, 65, 593–602. [Google Scholar] [CrossRef]
- Oguefai, T.; Yamasato, H.; Limmatvapirat, S.; Yonemochi, E.; Yamamoto, K. Structural change and complexation of strictly linear amloose induced by sealed-heaing with salicylyclic acid. J. Chem. Soc. Faraday Trans. 1998, 94, 923–927. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Chen, J.; Chen, L.; Zheng, B. Insight into the characterization and digestion of lotus seed starch-tea polyphenol complexes prepared under high hydrostatic pressure. Food Chem. 2019, 297, 124992. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bao, W.; Wu, Y.; Ouyang, J. Insights into the effects of caffeic acid and amylose on in vitro digestibility of maize starch-caffeic acid complex. Int. J. Biol. Macromol. 2020, 162, 922–930. [Google Scholar] [CrossRef]
- Lorentz, C.; Pencreac’H, G.; Soultani-Vigneron, S.; Rondeau-Mouro, C.; De Carvalho, M.; Pontoire, B.; Ergan, F.; Le Bail, P. Coupling lipophilization and amylose complexation to encapsulate chlorogenic acid. Carbohydr. Polym. 2012, 90, 152–158. [Google Scholar] [CrossRef]
- Le Bail, P.; Rondeau, C.; Buléon, A. Structural investigation of amylose complexes with small ligands: Helical conformation, crystalline structure and thermostability. Int. J. Biol. Macromol. 2005, 35, 1–7. [Google Scholar] [CrossRef]
- Lay Ma, U.V.; Floros, J.D.; Ziegler, G.R. Formation of inclusion complexes of starch with fatty acid esters of bioactive compounds. Carbohydr. Polym. 2011, 83, 1869–1878. [Google Scholar] [CrossRef]
- Uchino, T.; Tozuka, Y.; Oguchi, T.; Yamamoto, K. Inclusion compound formation of amylose by sealed-heating with salicylic acid analogues. J. Incl. Phenom. 2002, 43, 31–36. [Google Scholar] [CrossRef]
- Kenar, J.A.; Compton, D.L.; Little, J.A.; Peterson, S.C. Formation of inclusion complexes between high amylose starch and octadecyl ferulate via steam jet cooking. Carbohydr. Polym. 2016, 140, 246–252. [Google Scholar] [CrossRef]
- Fang, K.; He, W.; Jiang, Y.; Li, K.; Li, J. Preparation, characterization and physicochemical properties of cassava starch-ferulic acid complexes by mechanical activation. Int. J. Biol. Macromol. 2020, 160, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Lesmes, U.; Barchechath, J.; Shimoni, E. Continuous dual feed homogenization for the production of starch inclusion complexes for controlled release of nutrients. Innov. Food Sci. Emerg. Technol. 2008, 9, 507–515. [Google Scholar] [CrossRef]
- Lesmes, U.; Cohen, S.H.; Shener, Y.; Shimoni, E. Effects of long chain fatty acid unsaturation on the structure and controlled release properties of amylose complexes. Food Hydrocoll. 2009, 23, 667–675. [Google Scholar] [CrossRef]
- Wu, L.; Che, L.; Chen, X.D. Antiretrogradation in cooked starch-based product application of tea polyphenols. J. Food Sci. 2014, 79, E1984-90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and digestibility characterisation of maize starch–caffeic acid complexes. LWT 2020, 121, 108857. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Quek, S.; Perera, C.O. Physicochemical properties of bread dough and finished bread with added pectin fiber and phenolic antioxidants. J. Food Sci. 2011, 76, H97–H107. [Google Scholar] [CrossRef]
- Baiano, A.; Viggiani, I.; Terracone, C.; Romaniello, R.; Del Nobile, M.A. Physical and sensory properties of bread enriched with phenolic aqueous extracts from vegetable wastes. Czech J. Food Sci. 2015, 33, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- McCann, T.H.; Le Gall, M.; Day, L. Extensional dough rheology—Impact of flour composition and extension speed. J. Cereal Sci. 2016, 69, 228–237. [Google Scholar] [CrossRef]
- Labat, E.; Morel, M.H.; Rouau, X. Effects of laccase and ferulic acid on wheat flour doughs. Cereal Chem. 2000, 77, 823–828. [Google Scholar] [CrossRef]
- Koh, B.K.; Ng, P.K.W. Effects of ferulic acid and transglutaminase on hard wheat flour dough and bread. Cereal Chem. 2009, 86, 18–22. [Google Scholar] [CrossRef]
- Han, H.M.; Koh, B.K. Effect of phenolic acids on the rheological properties and proteins of hard wheat flour dough and bread. J. Sci. Food Agric. 2011, 91, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Snelders, J.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Impact of wheat bran derived arabinoxylanoligosaccharides and associated ferulic acid on dough and bread properties. J. Agric. Food Chem. 2014, 62, 7190–7199. [Google Scholar] [CrossRef] [PubMed]
- Nicks, F.; Richel, A.; Dubrowski, T.; Wathelet, B.; Wathelet, J.P.; Blecker, C.; Paquot, M. Effect of new synthetic PEGylated ferulic acids in comparison with ferulic acid and commercial surfactants on the properties of wheat flour dough and bread. J. Sci. Food Agric. 2013, 93, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Krekora, M.; Szymańska-Chargot, M.; Niewiadomski, Z.; Miś, A.; Nawrocka, A. Effect of cinnamic acid and its derivatives on structure of gluten proteins—A study on model dough with application of FT-Raman spectroscopy. Food Hydrocoll. 2020, 107, 105935. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Zhang, H.; Wang, J. Interactions between dietary fiber and ferulic acid changed the aggregation of gluten in a whole wheat model system. LWT 2018, 91, 55–62. [Google Scholar] [CrossRef]
- Jackson, G.M.; Hoseney, R.C. Fate of ferulic acid in overmixed wheat flour doughs: Partial characterization of a cysteine-ferulic acid adduct. J. Cereal Sci. 1986, 4, 87–95. [Google Scholar] [CrossRef]
- Painter, T.J.; Neukom, H. The Mechanism of Oxidative Gelation of a Glycoprotein from Wheatr Flour. Biochim. Biophys. Acta 1968, 158, 363–381. [Google Scholar] [CrossRef]
- Neukom, H. Chemistry and properties of the non-starch polysaccharides (NSP) of wheat flour. Leb. Wisschenschaft Technol. 1976, 9, 143–148. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Oxidative gelation studies of water-soluble pentosans from wheat. J. Cereal Sci. 1990, 11, 153–169. [Google Scholar] [CrossRef]
- Bettge, A.D.; Morris, C.F. Oxidative gelation measurement and influence on soft wheat batter viscosity and end-use quality. Cereal Chem. 2007, 84, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Piber, M.; Koehler, P. Identification of dehydro-ferulic acid-tyrosine in rye and wheat: Evidence for a covalent cross-link between arabinoxylans and proteins. J. Agric. Food Chem. 2005, 53, 5276–5284. [Google Scholar] [CrossRef] [PubMed]
- Konopka, I.; Tańska, M.; Faron, A.; Czaplicki, S. Release of free ferulic acid and changes in antioxidant properties during the wheat and rye bread making process. Food Sci. Biotechnol. 2014, 23, 831–840. [Google Scholar] [CrossRef]
- Dynkowska, W.M.; Cyran, M.R.; Ceglińska, A. Soluble and cell wall-bound phenolic acids and ferulic acid dehydrodimers in rye flour and five bread model systems: Insight into mechanisms of improved availability. J. Sci. Food Agric. 2015, 95, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Sęczyk, Ł.; Dziki, D.; Sikora, M. Interactions of green coffee bean phenolics with wheat bread matrix in a model of simulated in vitro digestion. Food Chem. 2018, 258, 301–307. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Skrajda-Brdak, M.; Konopka, I.; Tańska, M.; Czaplicki, S. Changes in the content of free phenolic acids and antioxidative capacity of wholemeal bread in relation to cereal species and fermentation type. Eur. Food Res. Technol. 2019, 245, 2247–2256. [Google Scholar] [CrossRef] [Green Version]
- Miflin, B.J. Extraction, Seperation and Polymorphism of the Prolamin Storage Proteins (secalins) of Rye. Cereal Chem. 1983, 60, 1–6. [Google Scholar]
- Kasarda, D.D.; Autran, J.C.; Lew, E.J.L.; Nimmo, C.C.; Shewry, P.R. N-terminal amino acid sequences of ω-gliadins and ω-secalins. Implications for the evolution of prolamin genes. Biochim. Biophys. Acta/Protein Struct. Mol. 1983, 747, 138–150. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S.; Pappin, D.J.; Keen, J. N-terminal amino acid sequences show that D-hordein of barley and high molecular weight (HMW) secalins of rye are homologous with HMW glutenin subunits of wheat. Cere 1988, 65, 510–511. [Google Scholar]
- Kipp, B.; Belitz, H.D.; Seilmeier, W.; Wieser, H. Comparative studies of high Mr subunits of rye and wheat. I. Isolation and biochemical characterisation and effects on gluten extensibility. J. Cereal Sci. 1996, 23, 227–234. [Google Scholar] [CrossRef]
- Gellrich, C.; Schieberle, P.; Wieser, H. Biochemical characterization and quantification of the storage protein (secalin) types in rye flour. Cereal Chem. 2003, 80, 102–109. [Google Scholar] [CrossRef]
- Gellrich, C.; Schieberle, P.; Wieser, H. Biochemical Characterization of γ-75 k Secalins of Rye I. Amino Acid Sequences. Cereal Chem. 2004, 81, 290–295. [Google Scholar] [CrossRef]
- Stȩpniewska, S.; Hassoon, W.H.; Szafrańska, A.; Cacak-Pietrzak, G.; Dziki, D. Procedures for breadmaking quality assessment of rye wholemeal flour. Foods 2019, 8, 331. [Google Scholar] [CrossRef] [Green Version]
- Oest, M.; Bindrich, U.; Voß, A.; Kaiser, H.; Rohn, S. Rye Bread Defects: Analysis of Composition and Further Influence Factors as Determinants of Dry-Baking. Foods 2020, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Banu, I.; Aprodu, I. Studies concerning the use of Lactobacillus helveticus and Kluyveromyces marxianus for rye sourdough fermentation. Eur. Food Res. Technol. 2012, 234, 769–777. [Google Scholar] [CrossRef]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M.; Pihlava, J.M.; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.A.; Chanliaud, E.; Rémésy, C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Požrl, T.; Kopjar, M.; Kurent, I.; Hribar, J.; Janeš, A.; Simčič, M. Phytate degradation during breadmaking: The influence of flour type and breadmaking procedures. Czech J. Food Sci. 2009, 27, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Rybka, K.; Sitarski, J.; Raczynska-Bojanowska, K. Ferulic Acid in Rye and Wheat Grain and Grain Dietary Fiber. Cereal Chem. 1993, 70, 55–59. [Google Scholar]
- Fretzdorff, B. Bestimmung der Peroxidase-Aktivität in Getreide. Leb.-Unters. Forsch. 1980, 170, 187–193. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D.; Gawlik-Dziki, U. Starch and protein analysis of wheat bread enriched with phenolics-rich sprouted wheat flour. Food Chem. 2017, 228, 643–648. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Yao, F.; Zhang, M.; Khalifa, I.; Li, K.; Li, C. Effect of persimmon tannin on the physicochemical properties of maize starch with different amylose/amylopectin ratios. Int. J. Biol. Macromol. 2019, 132, 1193–1199. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Q.; Chen, Z.; Xiao, H.; Science, F. The interaction between tea polyphenols and rice starch during gelatinization. Food Sci. Technol. Int. 2011, 17, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chen, Z.; Li, X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Liu, G.Q.; Yu, F. Evaluation of black tea polyphenol extract against the retrogradation of starches from various plant sources. Molecules 2012, 17, 8147–8158. [Google Scholar] [CrossRef]
- Beta, T.; Corke, H. Effect of Ferulic Acid and Catechin on Sorghum and Maize Starch Pasting Properties. Cereal Chem. 2004, 81, 418–422. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C. Resistant starches. Curr. Opin. Gastroenterol. 2000, 16, 178–183. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, Esterified, and Insoluble-Bound Phenolic Acids. Composition of Phenolic. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.J.; Yu, L. Effects of postharvest treatment and heat stress on availability of wheat antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef] [PubMed]
- Matissek, R.; Steiner, G.; Fischer, M. Lebensmittelanalytik; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; Volume 148, ISBN 978-3-54-092204-9. [Google Scholar]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C.G.; Bushuk, W. Comparison of the structure and composition of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991, 68, 139–144. [Google Scholar]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.A.; Micard, V.; de León, N.P.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Gan, Z.; Ellis, P.R.; Schofield, J.D. Gas Cell Stabilisation and Gas Retention in Wheat Bread Dough. J. Cereal Sci. 1995, 21, 215–230. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and endoxylanases in wheat flour bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Duodu, K.G. Effects of processing on antioxidant phenolics of cereal and legume grains. In ACS Symposium Series; Oxford University Press: Oxford, UK, 2011; Volume 1089, pp. 31–54. ISBN 978-0-84-122636-4. [Google Scholar]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of homogeneous fractions from wheat water-soluble arabinoxylans. Influence of the structure on their macromolecular characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef]
- Liukkonen, K.-H.; Katina, K.; Wilhelmsson, A.; Myllymäki, O.; Lampi, A.-M.; Kariluto, S.; Piironen, V.; Heinonen, S.-M.; Nurmi, T.; Adlercreutz, H.; et al. Process-indueced changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 2003, 62, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gaden, E.L. Fermentation process kinetics. Biotechnol. Bioeng. 2000, 67, 629–635. [Google Scholar] [CrossRef]
- Moore, J.; Luther, M.; Cheng, Z.; Yu, L. Effects of baking conditions, dough fermentation, and bran particle size on antioxidant properties of whole-wheat pizza crusts. J. Agric. Food Chem. 2009, 57, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Conde-Aguilera, J.A.; Haro, A.; Pastoriza de la Cueva, S.; Rufián-Henares, J.Á. A combined procedure to evaluate the global antioxidant response of bread. J. Cereal Sci. 2010, 52, 239–246. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Gélinas, P.; McKinnon, C.M. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int. J. Food Sci. Technol. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Technol. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Buksa, K.; Praznik, W.; Loeppert, R.; Nowotna, A. Characterization of water and alkali extractable arabinoxylan from wheat and rye under standardized conditions. J. Food Sci. Technol. 2016, 53, 1389–1398. [Google Scholar] [CrossRef] [Green Version]
- Boeriu, C.G.; Oudgenoeg, G.; Spekking, W.T.J.; Berendsen, L.B.J.M.; Vancon, L.; Boumans, H.; Gruppen, H.; VanBerkel, W.J.H.; Laane, C.; Voragen, A. Horseradish Peroxidase-Catalyzed Cross-Linking of Feruloylated Arabinoxylans with Casein. J. Agric. Food Chem. 2004, 52, 6633–6639. [Google Scholar] [CrossRef]
- Revanappa, S.B.; Salimath, P.V.; Prasada Rao, U.J.S. Effect of peroxidase on textural quality of dough and arabinoxylan characteristics isolated from whole wheat flour dough. Int. J. Food Prop. 2014, 17, 2131–2141. [Google Scholar] [CrossRef]
- Elofsson, U.; Eliasson, A.C.; Wahlgren, M.; Loosveld, A.M.A.; Courtin, C.M.; Delcour, J.A. Adsorption studies of interaction between water-extractable nonstarch polysaccharides and prolamins in cereals. Cereal Chem. 2000, 77, 679–684. [Google Scholar] [CrossRef]
- Renard, D.; Lavenant-Gourgeon, L.; Ralet, M.C.; Sanchez, C. Acacia senegal gum: Continuum of molecular species differing by their protein to sugar ratio, molecular weight, and charges. Biomacromolecules 2006, 7, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lv, P.; Zhang, L.; Yang, S.; Gao, Y. Structural and Functional Characterization of Laccase-Induced β-Lactoglobulin-Ferulic Acid-Chitosan Ternary Conjugates. J. Agric. Food Chem. 2019, 67, 12054–12060. [Google Scholar] [CrossRef] [PubMed]
- Ganzevles, R.A.; Zinoviadou, K.; Van Vliet, T.; Stuart, M.A.C.; De Jongh, H.H.J. Modulating surface rheology by electrostatic protein/polysaccharide interactions. Langmuir 2006, 22, 10089–10096. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Minaki, K.; Kobayashi, K. Improvement of Emulsifying Properties of Egg White Proteins by the Attachment of Polysaccharide through Maillard Reaction in a Dry State. J. Agric. Food Chem. 1993, 41, 540–543. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, Z.; Liu, L.; Lin, Y.; Wang, L.; Zhou, S. The effect of pentosanase on the solubilisation and degradation of arabinoxylan extracted from whole and refined wheat flour. J. Sci. Food Agric. 2017, 97, 1034–1041. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zheng, X.; Hong, J.; Bian, K.; Li, L. Interaction between A-type/B-type starch granules and gluten in dough during mixing. Food Chem. 2021, 358, 129870. [Google Scholar] [CrossRef]
- Cao, X.; Tong, J.; Ding, M.; Wang, K.; Wang, L.; Cheng, D.; Li, H.; Liu, A.; Liu, J.; Zhao, Z.; et al. Physicochemical properties of starch in relation to rheological properties of wheat dough (Triticum aestivum L.). Food Chem. 2019, 297, 125000. [Google Scholar] [CrossRef]
- Paulik, S.; Yu, W.W.; Flanagan, B.; Gilbert, R.G.; Jekle, M.; Becker, T. Characterizing the impact of starch and gluten-induced alterations on gelatinization behavior of physically modified model dough. Food Chem. 2019, 301, 125276. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, W.; Wang, L.; Ju, X. Insight into the effect of gluten-starch ratio on the properties of Chinese steamed bread (Mantou). Int. J. Biol. Macromol. 2020, 163, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, W.; Liu, Q.; Zhang, C.; Hu, H.; Zhang, H. Pasting, thermo, and Mixolab thermomechanical properties of potato starch–wheat gluten composite systems. Food Sci. Nutr. 2020, 8, 2279–2287. [Google Scholar] [CrossRef] [PubMed]










| K-Value [mol−1 dm3] | pH (Temp) | Method | References |
|---|---|---|---|
| 1.793 × 104 25.47 × 104 80.68 × 104 | 7.2 (25 °C) 7.2 (35 °C) 7.2 (45 °C) | Fluorescence quenching (tryptophan) | [69] |
| 17.24 × 103 4.01 × 103 | 4.8 (25 °C) 7.0 (25 °C) | Hummel–Dreyer method (HD) | [23] |
| 5 × 103 5.56 × 104 | 4.8 (T n.a.) 7.0 (T n.a.) | Fluorescence quenching (tryptophan) | [68] |
| 40.15 × 104 | 7.4 (25 °C) | Fluorescence quenching (tryptophan) | [37] |
| 5.56 × 104 5.09 × 104 2.49 × 104 | 7.8 (20 °C) 7.8 (25 °C) 7.8 (25 °C) | Affinity capillary electrophoresis (ACE) Fluorescence quenching (tryptophan) | [71] |
| 5.1 × 104 | 8.5 (T n.a.) | Surface plasmon resonance (SPR) | [70] |
| Effect | FA Added | References |
|---|---|---|
| Increased dough strength Reduction of mixing time | 0.034 g/100 g | [145] |
| Increased amount of SDS-soluble proteins in dough Reduction of bread volume Reduction of mixing time Decreased dough strength Increased crumb hardness | 0.025 g/100 g | [147] |
| Increased number of SDS-soluble proteins in dough Reduction of bread volume Increased crumb hardness | 0.0825–0.5 g/100 g | [149] |
| Decreased amount of disulfide bonds No influence on bread volume | 0.2–1.8 g/100 g FA/eq. AXOS-FA | [148] |
| Decreased amount of disulfide bonds Decreased amount of free thiol groups Decreased denaturation enthalpy and temperature of gluten proteins | 1–4 g/100 g | [151] |
| Decreased amount of disulfide bonds Increased amount of free thiol groups | 0.05–0.2 g/100 g | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. https://doi.org/10.3390/foods10112798
Schefer S, Oest M, Rohn S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods. 2021; 10(11):2798. https://doi.org/10.3390/foods10112798
Chicago/Turabian StyleSchefer, Simone, Marie Oest, and Sascha Rohn. 2021. "Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties" Foods 10, no. 11: 2798. https://doi.org/10.3390/foods10112798
APA StyleSchefer, S., Oest, M., & Rohn, S. (2021). Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods, 10(11), 2798. https://doi.org/10.3390/foods10112798