Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Overview
2.2. Subjects
2.3. Test of Olfactory Capabilities
2.4. PROP Status
2.5. Liking and Overall Flavor Intensity in Real Food Products
2.6. Food Neophobia Assessment
2.7. Data Analysis
3. Results
3.1. Subjects Description
3.2. Relationships among Individual Variables
3.3. Recognition, Liking, and Intensity Perception of Odors
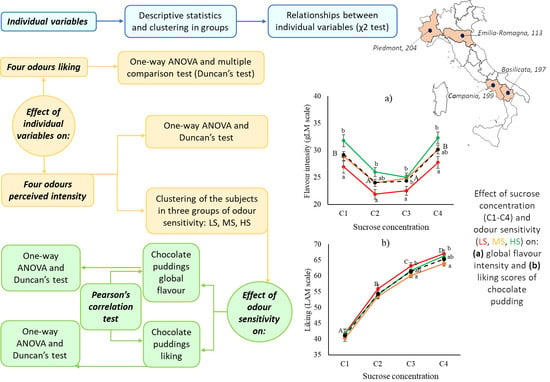
3.4. Effect of Odor Sensitivity on Global Flavor Perception and Liking in Real Food Products
4. Discussion
4.1. Relationships among Individual Variables
4.2. Recognition, Liking, and Intensity Perception of Odors
4.3. Effect of Odor Sensitivity on Global Flavor Perception and Liking in a Real Product
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludvigson, H.W.; Rottman, T.R. Effects of ambient odors of lavender and cloves on cognition, memory, affect and mood. Chem. Senses 1989, 14, 525–536. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croy, I.; Bendas, J.; Wittrodt, N.; Lenk, M.; Joraschky, P.; Weidner, K. Gender-specific relation between olfactory sensitivity and disgust perception. Chem. Senses 2017, 42, 487–492. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Case, T.I.; Oaten, M.J. Frequency and recency of infection and their relationship with disgust and contamination sensitivity. Evol. Hum. Behav. 2009, 30, 363–368. [Google Scholar] [CrossRef]
- Rozin, P. Taste–smell confusions and the duality of the olfactory sense. Percept. Psychophys. 1982, 31, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.; Craven, B.; Khan, R.M.; Chang, S.J.; Kang, I.; Judkewitz, B.; Volpe, J.; Settles, G.; Sobel, N. Mechanisms of scent-tracking in humans. Nat. Neurosci. 2007, 10, 27–29. [Google Scholar] [CrossRef]
- Boesveldt, S.; Frasnelli, J.; Gordon, A.R.; Lundström, J.N. The fish is bad: Negative food odors elicit faster and more accurate reactions. Biol. Psychol. 2010, 82, 313–317. [Google Scholar] [CrossRef]
- Yamada, Y.; Sasaki, K.; Kunieda, S.; Wada, Y. Scents boost preference for novel fruits. Appetite 2014, 81, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R. Olfactory influences on appetite and satiety in humans. Physiol. Behav. 2006, 87, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Boesveldt, S.; Bobowski, N.; McCrickerd, K.; Maître, I.; Sulmont-Rossé, C.; Forde, C.G. The changing role of the senses in food choice and food intake across the lifespan. Food Qual. Prefer. 2018, 68, 80–89. [Google Scholar] [CrossRef]
- Gaillet-Torrent, M.; Sulmont-Rossé, C.; Issanchou, S.; Chabanet, C.; Chambaron, S. Impact of a non-attentively perceived odour on subsequent food choices. Appetite 2014, 76, 17–22. [Google Scholar] [CrossRef]
- Ramaekers, M.G.; Boesveldt, S.; Gort, G.; Lakemond, C.M.M.; Van Boekel, M.A.J.S.; Luning, P.A. Sensory-specific appetite is affected by actively smelled foododors and remains stable over time in normal-weight women. J. Nutr. 2014, 144, 1314–1319. [Google Scholar] [CrossRef] [Green Version]
- Morquecho-Campos, P.; Graaf, K.D.; Boesveldt, S. Smelling our appetite? The influence of food odors on congruent appetite, food preferences and intake. Food Qual. Prefer. 2020, 85, 103959. [Google Scholar] [CrossRef]
- Dicker, D.; Beck, A.; Markel, A.; Marcovicu, D.; Mazzawi, S.; Sarid, M.; Greenberg, E.; Atkinson, R. Weight loss, dietary preferences, and reduction in the sense of smell with the use of a novel nasal device. Obes. Facts 2020, 13, 473–486. [Google Scholar] [CrossRef]
- Rasmussen, V.F.; Vestergaard, E.T.; Hejlesen, O.; Andersson, C.U.N.; Cichosz, S.L. Prevalence of taste and smell impairment in adults with diabetes: A cross-sectional analysis of data from the National Health and Nutrition Examination Survey (NHANES). Prim. Care Diabetes 2018, 12, 453–459. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Sobel, N. An odor is not worth a thousand words: From multidimensional odors to unidimensional odor objects. Annu. Rev. Psychol. 2010, 61, 219–241. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.A.; Stevenson, R.J. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003, 26, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Dematté, M.L.; Endrizzi, I.; Biasioli, F.; Corollaro, M.L.; Pojer, N.; Zampini, M.; Aprea, E.; Gasperi, F. Food neophobia and its relation with olfactory ability in common odour identification. Appetite 2013, 68, 112–117. [Google Scholar] [CrossRef]
- Demattè, M.L.; Endrizzi, I.; Gasperi, F. Food neophobia and its relation with olfaction. Front. Psychol. 2014, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher body mass index is associated with subjective olfactory dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef] [Green Version]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: Changes with age. Science 1984, 226, 1441–1443. [Google Scholar] [CrossRef]
- Cain, W.S.; Gent, J.F. Olfactory sensitivity: Reliability, generality, and association with aging. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Miwa, T.; Furukawa, M.; Tsukatani, T.; Costanzo, R.M.; Dinardo, L.J.; Reiter, E.R. Impact of olfactory impairment quality of life and disability. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, N.P.; Roberts, S.B. The anorexia of aging in humans. Physiol. Behave 2006, 88, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living elderly women. J. Am. Diet. Assoc. 1995, 95, 879–884. [Google Scholar] [CrossRef]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef]
- Palouzier-Paulignan, B.; Lacroix, M.C.; Aime, P.; Bally, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef]
- Brünner, Y.F.; Benedict, C.; Freiherr, J. Intranasal insulin reduces olfactory sensitivity in normosmic humans. J. Clin. Endocrinol. Metab. 2013, 98, E1626–E1630. [Google Scholar] [CrossRef] [Green Version]
- Poessel, M.; Freiherr, J.; Wiencke, K.; Villringer, A.; Horstmann, A. Insulin resistance is associated with reduced food odor sensitivity across awide range of bodyweights. Nutrients 2020, 12, 2201. [Google Scholar] [CrossRef]
- Richardson, B.E.; Vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered olfactory acuity in the morbidly obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef]
- Jurowich, C.F.; Seyfried, F.; Miras, A.D.; Bueter, M.; Deckelmann, J.; Fassnacht, M.; Germer, C.T.; Thalheimer, A. Does bariatric surgery change olfactory perception? Results of the early postoperative course. Int. J. Colorectal Dis. 2014, 29, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D.; Whittle, A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef]
- Hedner, M.; Larsson, M.; Arnold, N.; Zucco, G.M.; Hummel, T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 2010, 32, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009, 97, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Kern, D.W.; Wroblewski, K.E.; Schumm, L.P.; Pinto, J.M.; Chen, R.C.; McClintock, M.K. Olfactory function in Wave 2 of the national social life, health, and aging project. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2014, 69, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokowska, V.A.; Schriever, V.A.; Gudziol, V.; Hummel, C.; Hähner, A.; Iannilli, E.; Sinding, C.; Aziz, M.; Seo, H.S.; Negoias, S.; et al. Changes of olfactory abilities in relation to age: Odor identification in more than 1400 people aged 4 to 80 years. Eur. Arch. Oto Rhino Laryngol. 2014, 272, 1937–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudenbush, B.; Schroth, F.; Reilley, S.; Frank, R.A. Food neophobia, odor evaluation and exploratory sniffing behaviour. Appetite 1998, 31, 171–183. [Google Scholar] [CrossRef]
- Prescott, J.; Burns, J.; Frank, R.A. Influence of odor hedonics, food-relatedness, and motivational state on human sniffing. Chemosens. Percept. 2010, 3, 85–90. [Google Scholar] [CrossRef]
- Menghi, L.; Khomenko, I.; Pedrotti, M.; Danny, C.; Eugenio, A.; Isabella, E.; Annachiara, C.; Franco, B.; Davide, G.; Flavia, G. Arousal influences olfactory abilities in adults with different degree of food neophobia. Sci. Rep. 2020, 10, 20538. [Google Scholar] [CrossRef]
- Flaherti, T.J.; Lim, J. Individual differences in retronasal odor responsiveness: Effects of aging and concurrent taste. Chem. Percept. 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavour perception. Lead. Edge 2015, 23, 365–369. [Google Scholar]
- Small, D.M.; Jones-Gotman, M.; Zatorre, R.J.; Petrides, M.; Evans, A.C. Flavor processing: More than the sum of its parts. NeuroReport 1997, 22, 3913–3917. [Google Scholar] [CrossRef]
- Piochi, M.; Dinnella, C.; Spinelli, S.; Monteleone, E.; Torri, L. Individual differences in responsiveness to oral sensations and odours with chemesthetic activity: Relationships between sensory modalities and impact on the hedonic response. Food Qual. Prefer. 2021, 88, 104–112. [Google Scholar] [CrossRef]
- Hayes, J.E.; Keast, R.S.J. Two decades of supertasting: Where do we stand? Physiol. Behav. 2011, 104, 1072–1074. [Google Scholar] [CrossRef] [Green Version]
- Tomassini Barbarossa, I.; Ozdener, M.H.; Melis, M.; Love-Gregory, L.; Mitreva, M.; Abumrad, N.A.; Pepino, M.Y. Variant in a common odorant-binding protein gene is associated with bitter sensitivity in people. Behav. Brain Res. 2017, 329, 200–204. [Google Scholar] [CrossRef]
- Yackinous, C.; Guinard, J.X. Relation between PROP taster status and fat perception, touch, and olfaction. Physiol. Behav. 2001, 72, 427–437. [Google Scholar] [CrossRef]
- Jaeger, S.; McRae, J.F.; Bava, C.M.; Beresford, M.K.; Hunter, D.; Jia, Y.; Chheang, S.L.; Jin, D.; Peng, M.; Gamble, J.C.; et al. A mendelian trait for olfactory sensitivity affects odor experience and food selection. Curr. Biol. 2013, 23, 1601–1605. [Google Scholar] [CrossRef] [Green Version]
- Piochi, M.; Cabrino, G.; Morini, G.; Torri, L. Individual differences in the perception of orthonasal irritation induced by food. Appetite 2020, 144, 104460. [Google Scholar] [CrossRef]
- Monteleone, E.; Spinelli, S.; Dinnella, C.; Endrizzi, I.; Laureati, M.; Pagliarini, E.; Sinesio, F.; Gasperi, F.; Torri, L.; Aprea, E.; et al. Exploring influences on food choice in a large population sample: The Italian taste project. Food Qual. Prefer. 2017, 59, 123–140. [Google Scholar] [CrossRef]
- Weyer-Menkhoff, I.; Lötsch, J. Human pharmacological approaches to TRP- ion-channel-based analgesic drug development. Drug Discov. 2018, 23, 2003–2012. [Google Scholar] [CrossRef]
- Richards, P.M.; Johnson, E.C.; Silver, W.L. Four irritating odorants target the trigeminal chemoreceptor TRPA. Chemosens. Percept. 2010, 3, 190–199. [Google Scholar] [CrossRef]
- Joussain, P.; Bessy, M.; Faure, F.; Bellil, D.; Landis, B.N.; Hugentobler, M.; Tuorila, H.; Mustonen, S.; Vento, S.I.; Delphin-Combe, F.; et al. Application of the European Test of Olfactory Capabilities in patients with olfactory impairment. Eur. Arch. Oto Rhino Laryngol. 2015, 273, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The GLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef]
- Schutz, H.G.; Cardello, A.V. A labeled affective magnitude (LAM) scale for assessing food liking/disliking. J. Sens. Stud. 2001, 16, 117–159. [Google Scholar] [CrossRef]
- Pliner, P.; Hobden, K. Development of a scale to measure the trait of food neophobia in humans. Appetite 1992, 19, 105–120. [Google Scholar] [CrossRef]
- Laureati, M.; Spinelli, S.; Monteleone, E.; Dinnella, C.; Prescott, J.; Cattaneo, C.; Pagliarini, E. Associations between food neophobia and responsiveness to “warning” chemosensory sensations in food products in a large population sample. Food Qual. Prefer. 2018, 68, 113–124. [Google Scholar] [CrossRef]
- Li, K.K.; Concepcion, R.Y.; Lee, H.; Cardinal, B.J.; Ebbeck, V.; Woekel, E.; Readdy, R.T. An examination of sex differences in relation to the eating habits and nutrient intakes of university students. J. Nutr. Educ. Behav. 2012, 44, 246–250. [Google Scholar] [CrossRef]
- Leblanc, V.; Begin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in dietary intakes: What is the contribution of motivational variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Del Papa, S. Dietary habits and gender differences. Ital. J. Gend. Specif. Med. 2017, 3, 55–58. [Google Scholar]
- El-Hazmi, M.A.F.; Warsy, A. Relationship between age and the prevalence of obesity and overweight in saudi population. Bahrain Med. Bull. 2002, 24, 1–7. [Google Scholar]
- Gurney, M.; Gorstein, J. The global prevalence of obesity: An initial overview of available data. World Heal. Stat. Q. Rapp. Trimest. Stat. Sanit. Mond. 1988, 41, 251–254. [Google Scholar]
- Epstein, F.H.; Higgins, M. Epidemiology of obesity. In Obesity; Bjorntorp, P., Brodorff, B.N., Eds.; Lippincott Company: Philadelphia, PA, USA, 1992; pp. 230–342. [Google Scholar]
- Stafford, L.D.; Welbeck, K. High hunger state increases olfactory sensitivity to neutral but not food odors. Chem. Senses 2011, 36, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, B.J.; Melis, M.; Koelliker, Y.; Gasparini, P.; Ahijevych, K.L.; Barbarossa, I.T. Ahijevych, and iole tomassini barbarossa. factors influencing the phenotypic characterization of the oral marker, PROP. Nutrients 2017, 9, 1275. [Google Scholar] [CrossRef] [Green Version]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Prutkin, J.; Fisher, E.M.; Etter, L.; Fast, K.; Gardner, E.; Lucchina, L.A.; Snyder, D.J.; Tie, K.; Weiffenbach, J.; Bartoshuk, L.M. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol. Behav. 2000, 69, 161–173. [Google Scholar] [CrossRef]
- Tepper, B.J.; Nurse, R.J. PROP taster status is related to the perception and preference for fat. Ann. N. Y. Acad. Sci. 1998, 855, 802–804. [Google Scholar] [CrossRef]
- Hayes, J.E.; Sullivan, B.S.; Duffy, V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010, 100, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.E.; Cruickshanks, K.J.; Schubert, C.R.; Pinto, A.; Klein, R.; Pankratz, N.; Pankow, J.S.; Huang, G.H. Factors related to fungiform papillae density: The beaver dam offspring study. Chem. Senses 2013, 38, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, J.R.; Davis, S.H.; Englehardt, R.A.; Miller, I.J.; Schiffrman, S.S.; Phillips, C. Taste performance on the anterior human tongue varles with fungiform taste bud density. Chem. Senses 1993, 18, 449–460. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Tepper, B.J.; Rietzschel, J.; Prescott, J. Human hedonic responses to sweetness: Role of taste genetics and anatomy. Physiol. Behav. 2007, 91, 264–273. [Google Scholar] [CrossRef]
- Correa, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Changes in fungiform papillae density during development in humans. Chem. Senses 2013, 38, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Steele, C.M.; Hill, L.; Stokely, S.; Peladeau-Pigeon, M. Age and strength influences on lingual tactile acuity. J. Texture Stud. 2014, 45, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Segovia, C.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. A quantitative study of fungiform papillae and taste pore density in adults and children. Brain Res. Dev. Brain Res. 2002, 138, 135–146. [Google Scholar] [CrossRef]
- Fekete, T.; Židek, R.; Šnirc, M.; Belej, L. Effect of ageing process on fungiform papillae density. Acta Fytotechn Zootech. 2017, 20, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Huang, L.; Wang, H. Taste bud homeostasis in health, disease, and aging. Chem. Senses 2014, 39, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Psychophysical dissection of genotype effects on human bitter perception. Chem. Senses 2010, 36, 161–167. [Google Scholar] [CrossRef]
- Von Atzingen, M.C.B.C.; Silva, M.E.M.P. 6-n-propyltiouracil (PROP) taster status in Brazilian adults. Food Sci. Technol. 2012, 32, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Nordin, S.; Broman, D.A.; Garvill, J.; Nyroos, M. Gender differences in factors affecting rejection of food in healthy young Swedish adults. Appetite 2004, 43, 295–301. [Google Scholar] [CrossRef]
- Meiselman, H.L.; King, S.C.; Gillette, M. The demographics of neophobia in a large commercial US sample. Food Qual. Prefer. 2010, 21, 893–897. [Google Scholar] [CrossRef]
- Siegrist, M.; Hartmann, C.; Keller, C. Antecedents of food neophobia and its association with eating behavior and food choices. Food Qual. Prefer. 2013, 30, 293–298. [Google Scholar] [CrossRef]
- Hursti, U.K.K.; Sjoden, P.O. Food and general neophobia and their relationship with self-reported food choice. Familial resemblance in Swedish families with children of ages 7–17 years. Appetite 1997, 29, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, U.K.; Sjoden, P.O. Food and general neophobia in Swedish families: Parent-child comparisons and relationships with serving specific foods. Appetite 1996, 26, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tuorila, H.M.; Meiselman, H.L.; Cardello, A.V.; Lesher, L.L. Effect of expectations and the definition of product category on the acceptance of unfamiliar foods. Food Qual. Prefer. 1998, 9, 421–430. [Google Scholar] [CrossRef]
- Hartmann, C.; Dohle, S.; Siegrist, M. Importance of cooking skills form balanced food choices. Appetite 2013, 65, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P.; Pelchat, M.; Grabski, M. Reduction of neophobia in humans by exposure to novel foods. Appetite 1993, 20, 111–123. [Google Scholar] [CrossRef]
- Tuorila, H.; Lähteenmäki, L.; Pohjalainen, L.; Lotti, L. Food neophobia among the Finns and related responses to familiar and unfamiliar foods. Food Qual. Prefer. 2001, 12, 29–37. [Google Scholar] [CrossRef]
- Mascarello, G.; Pinto, A.; Rizzoli, V.; Tiozzo, B.; Crovato, S.; Ravarotto, L. Ethnic food consumption in Italy: The role of food neophobia and openness to different cultures. Foods 2020, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Doty, R.L. Handbook of Olfaction and Gustation, 3rd ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ehrlichman, H.; Bastone, L. Olfaction and emotion. In Science of Olfaction; Serby, M., Chobor, K., Eds.; Springer: New York, NY, USA, 1990; pp. 410–438. [Google Scholar]
- Rouby, C.; Pouliot, S.; Bensafi, M. Odor hedonics and their modulators. Food Qual. Prefer. 2009, 8, 545–549. [Google Scholar] [CrossRef]
- Schab, F.R. Odor memory: Taking stock. Psychol. Bull. 1991, 109, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Rouby, C.; Schaal, B.; Dubois, D.; Gervais, R.; Holley, A. Olfaction, Taste and Cognition; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Larsson, M.; Backman, L. Semantic mediation of age related deficits in episodic recognition of common odors. Ann. N. Y. Acad. Sci. 1998, 855, 675–680. [Google Scholar] [CrossRef]
- Bensafi, M.; Rouby, C. Individual differences in odor imaging ability reflect differences in olfactory and emotional perception. Chem. Senses 2007, 32, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, J.; Zatorre, R.J.; Petrides, M.; Jones Gotman, M. The mind’s nose: Effects of odor and visual imagery on odor detection. Psychol. Sci. 2004, 15, 143–148. [Google Scholar] [CrossRef]
- Rinck, F.; Rouby, C.; Bensafi, M. Which format for odor images? Chem. Senses 2009, 34, 11–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouby, C.; Bourgeat, F.; Rinck, F.; Poncelet, J.; Bensafi, M. Perceptual and sensorimotor differences between “good” and “poor” olfactory mental imagers. Ann. N. Y. Acad. Sci. 2009, 1170, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.K.; Christoff, K.; Stappen, I.; Panitz, D.; Ghahremani, D.G.; Glover, G.; Gabrieli, J.D.E.; Sobel, N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003, 6, 196–202. [Google Scholar] [CrossRef]
- Prescott, J.; Kim, H.; Kim, K.O. Cognitive mediation of hedonic changes to odors following exposure. Chemosens. Percept. 2008, 1, 2–8. [Google Scholar] [CrossRef]
- Stagnetto, J.; Rouby, C.; Bensafi, M. Contextual cues during olfactory learning improve memory for smells in children. Eur. Rev. Appl. Psychol. 2006, 56, 253–259. [Google Scholar] [CrossRef]
- Rolls, E.T.; Rolls, J.H. Olfactory sensory specific satiety in humans. Physiol. Behav. 1997, 61, 461–473. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Hummel, T.; Larsson, M. Identification of unpleasant odors is independent of age. Arch. Clin. Neuropsychol. 2006, 21, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Pouliot, S.; Bourgeat, F.; Barkat, S.; Rouby, C.; Bensafi, M. Increase in anhedonia level in menopausal women is accompanied by a shift in olfactory function. Chemosens. Percept. 2008, 1, 43–47. [Google Scholar] [CrossRef]
- Barkat, S.; Poncelet, J.; Landis, B.N.; Rouby, C.; Bensafi, M. Improved smell pleasantness after odor-taste associative learning in humans. Neurosci. Lett. 2008, 434, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.; Hummel, T. A new method for comparing orthonasal and retronasal olfaction. Behav. Neurosci. 2004, 118, 412–419. [Google Scholar] [CrossRef]
- Ishii, A.; Roudnitzky, N.; Beno, N.; Bensafi, M.; Hummel, T.; Rouby, C.; Danguin, T. Synergy and masking in odor mixtures: An electrophysiological study of orthonasal vs. retronasal perception. Chem. Senses 2008, 33, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Gerber, J.C.; Mak, Y.E.; Hummel, T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 2005, 47, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayabe Kanamura, S.; Schicker, I.; Laska, M.; Hudson, R.; Distel, H.; Kobayakawa, T.; Saito, S. Differences in perception of everyday odors: A Japanese-German cross cultural study. Chem. Senses 1998, 23, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Wysocki, C.J.; Pierce, J.D.; Gilbert, A.N. Geographic, cross cultural, and individual variation in human olfaction. In Smell and Taste in Health and Disease; Getchell, T.V., Doty, T.V., Bartoshuck, L.M., Snow, J.B., Eds.; Raven Press: New York, NY, USA, 1991; pp. 287–314. [Google Scholar]
- Chrea, C.; Valentin, D.; Rossé, S.; May, H.; Nguyen, H.; Abdi, H. Culture and odor categorization: Agreement between cultures depends upon the odors. Food Qual. Prefer. 2004, 15, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Koelega, H.S. Sex differences in olfactory sensitivity and the problem of the generality of smell acuity. Percept. Mot. Skills 1994, 78, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Palisch, K.; Wolf, S.R.; Meyer, E.D.; Hüttenbrink, K.B.; Roscher, S.; Wagner, R.; Hummel, T. A threshold like measure for the assessment of olfactory sensitivity: The “random” procedure. Eur. Arch. Oto Rhino Laryngol. 2001, 258, 168–172. [Google Scholar] [CrossRef]
- Herz, R.S.; Inzlicht, M. Sex differences in response to physical and social factors involved in human mate selection. The importance of smell for women. Evol. Hum. Behav. 2002, 23, 359–364. [Google Scholar] [CrossRef]
- Havlicek, J.; Saxton, T.K.; Roberts, S.C.; Jozifkova, E.; Lhota, S.; Valentova, J.; Flegr, J. He sees, she smells? Male and female reports of sensory reliance in mate choice and non mate choice contexts. Pers. Indiv. Differ. 2008, 45, 565–570. [Google Scholar] [CrossRef]
- Schleidt, M.; Hold, B.; Attili, G. A cross cultural study on the attitude towards personal odors. J. Chem. Ecol. 1981, 7, 19–31. [Google Scholar] [CrossRef]
- Larsson, M.; Lövdén, M.; Nilsson, L.G. Sex differences in recollective experience for olfactory and verbal information. Acta Psychol. 2003, 112, 89–103. [Google Scholar] [CrossRef]
- Oliveira Pinto, A.V.; Santos, R.M.; Coutinho, R.A.; Oliveira, L.M.; Santos, G.B.; Alho, A.T.; Leite, R.E.; Farfel, J.M.; Suemoto, C.K.; Grinberg, L.T.; et al. Sexual dimorphism in the human olfactory bulb: Females have more neurons and glial cells than males. PLoS ONE 2014, 9, 111–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Eslinger, P.J.; Smith, M.B.; Yang, Q.X. Functional magnetic resonance imaging study of human olfaction and normal aging. J. Gerontol. A BiolSci. Med. Sci. 2005, 60, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Markovic, K.; Reulbach, U.; Vassiliadu, A.; Lunkenheimer, J.; Lunkenheimer, B.; Spannenberger, R.; Thuerauf, N. Good news for elderly persons: Olfactory pleasure increases at later stages of the life span. J. Gerontol. A BiolSci. Med. Sci. 2007, 62, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.C.; Cain, W.S. Smelling via the mouth: Effect of aging. Percept. Psychophys. 1986, 40, 142–146. [Google Scholar] [CrossRef]
- Drewnowski, A.; Henderson, S.A.; Barratt Fornell, A. Genetic sensitivity to 6 n propylthiouracil and sensory responses to sugar and fat mixtures. Physiol. Behav. 1998, 63, 771–777. [Google Scholar] [CrossRef]
- Looy, H.; Weingarten, H.P. Facial expressions and genetic sensitivity to 6 normal propylthiouracil predict hedonic response to sweet. Physiol. Behav. 1992, 52, 75–82. [Google Scholar] [CrossRef]
- Bartoshuk, L.M. Comparing sensory experiences across individuals: Recent psychophysical advances illuminate genetic variation in taste perception. Chem. Senses 2000, 25, 447–460. [Google Scholar] [CrossRef]
- Prescott, J.; Swain Campbell, N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in prop tasters and nontasters. Chem. Senses 2000, 25, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Laing, D.G. Optimum perception of odor intensity by humans. Physiol. Behav. 1985, 34, 569–574. [Google Scholar] [CrossRef]
- Lo, C.; Arora, S.; Shlomo, Y.; Barber, T.R.; Lawton, M.; Klein, J.C.; Kanavou, S.; Janzen, A.; Sittig, E.; Oertel, W.H.; et al. Olfactory testing in parkinson disease and REM behavior disorder a machine learning approach. Neurology 2021, 96, 2016–2027. [Google Scholar] [CrossRef]
- Limphaibool, N.; Iwanowski, P.; Kozubski, W.; Swidziński, T.; Frankowska, A.; Kamińska, I.; Linkowska-Swidzińska, K.; Sekula, A.; Swidziński, P.; Maciejewska-Szaniec, Z.; et al. Subjective and objective assessments of post-traumatic olfactory dysfunction. Front. Neurol. 2020, 11, 970. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Wang, K.; Goldberg, J.; Aliani, M. Factors affecting the ortho- and retronasal perception of flavors: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Delime, P.; O’Mahony, K.; Desforges, N.; Taylor, A.J.; Hort, J. Comparing the relative sensitivity of ortho and retronasal perception of a strawberry flavour model using omission testing. Flavour Fragr. J. 2016, 31, 377–384. [Google Scholar] [CrossRef]
- Sollai, G.; Tomassini Barbarossa, I.; Usai, P.; Hummel, T.; Crnjar, R. Association between human olfactory performance and ability to detect single compounds in complex chemical mixtures. Physiol. Behav. 2020, 217, 112820. [Google Scholar] [CrossRef]







| Number of Subjects | Females (n = 420) | Males (n = 329) | |
|---|---|---|---|
| Provenance | |||
| Emilia-Romagna | 113 | 73 (65%) | 40 (35%) |
| Piedmont | 240 | 125 (52%) | 115 (48%) |
| Campania | 199 | 110 (55%) | 89 (45%) |
| Basilicata | 197 | 112 (57%) | 85 (43%) |
| Age (years) | |||
| 19–30 | 269 | 155 (58%) | 114 (42%) |
| 31–45 | 249 | 134 (54%) | 115 (46%) |
| 46–65 | 231 | 131 (57%) | 100 (43%) |
| Body mass index 1 | |||
| Underweight | 31 | 27 (87%) | 4 (13%) |
| Normal-weight | 447 | 284 (63%) | 163 (37%) |
| Overweight | 209 | 79 (38%) | 130 (62%) |
| Obese | 62 | 29 (47%) | 33 (53%) |
| Smoking habits | |||
| Non-smoker | 404 | 234 (58%) | 170 (42%) |
| Ex-smoker | 137 | 70 (51%) | 68 (49%) |
| Current-smoker | 206 | 115 (56%) | 91 (44%) |
| Self-reported smell | |||
| <Normal | 27 | 11 (41%) | 16 (59%) |
| Normal | 620 | 348 (56%) | 272 (44%) |
| >Normal | 102 | 61 (60%) | 41 (40%) |
| Odor sensitivity | |||
| Low-sensitivity (LS) | 187 | 83 (44%) | 104 (56%) |
| Moderate-sensitivity (MS) | 349 | 142 (41%) | 349 (59%) |
| High-sensitivity (HS) | 213 | 130 (61%) | 83 (39%) |
| PROP status | |||
| No-taster (NT) | 160 | 81 (51%) | 79 (49%) |
| Medium-taster (MT) | 331 | 168 (51%) | 163 (49%) |
| Super-taster (ST) | 258 | 171 (66%) | 87 (34%) |
| FNS | |||
| Low-Neophobia (LN) | 203 | 131 (65%) | 72 (35%) |
| Medium-Neophobia (MN) | 347 | 173 (50%) | 174 (50%) |
| High-Neophobia (HN) | 199 | 116 (58%) | 83 (42%) |
| Individual Variables | Gender | Age | BMI | SH 1 | SRS 2 | PROP | FNS | Provenance |
|---|---|---|---|---|---|---|---|---|
| Gender | n.d. | |||||||
| Age | 0.672 | n.d. | ||||||
| BMI | <0.0001 | 0.005 | n.d. | |||||
| SH1 | 0.339 | <0.0001 | 0.422 | n.d. | ||||
| SRS | 0.146 | 0.436 | 0.012 | 0.192 | n.d. | |||
| PROP | 0.0001 | 0.025 | 0.163 | 0.494 | 0.577 | n.d. | ||
| FNS | 0.003 | <0.0001 | 0.068 | 0.112 | 0.092 | 0.430 | n.d. | |
| Provenance | 0.162 | <0.0001 | <0.0001 | <0.0001 | 0.087 | 0.0001 | <0.0001 | n.d. |
| Anise | Banana | Mint | Pine | |
|---|---|---|---|---|
| Recognition (%) | ||||
| Yes | 75 | 92 | 99 | 88 |
| No | 25 | 8 | 1 | 12 |
| Liking | 6 ± 2 | 6 ± 2 | 7 ± 2 | 6 ± 2 |
| Intensity | 4 ± 3 | 5 ± 2 | 6 ± 2 | 5 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puleo, S.; Braghieri, A.; Pacelli, C.; Bendini, A.; Toschi, T.G.; Torri, L.; Piochi, M.; Di Monaco, R. Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food. Foods 2021, 10, 3122. https://doi.org/10.3390/foods10123122
Puleo S, Braghieri A, Pacelli C, Bendini A, Toschi TG, Torri L, Piochi M, Di Monaco R. Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food. Foods. 2021; 10(12):3122. https://doi.org/10.3390/foods10123122
Chicago/Turabian StylePuleo, Sharon, Ada Braghieri, Corrado Pacelli, Alessandra Bendini, Tullia Gallina Toschi, Luisa Torri, Maria Piochi, and Rossella Di Monaco. 2021. "Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food" Foods 10, no. 12: 3122. https://doi.org/10.3390/foods10123122
APA StylePuleo, S., Braghieri, A., Pacelli, C., Bendini, A., Toschi, T. G., Torri, L., Piochi, M., & Di Monaco, R. (2021). Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food. Foods, 10(12), 3122. https://doi.org/10.3390/foods10123122