Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines
Abstract
:1. Introduction
2. Materials and Methods
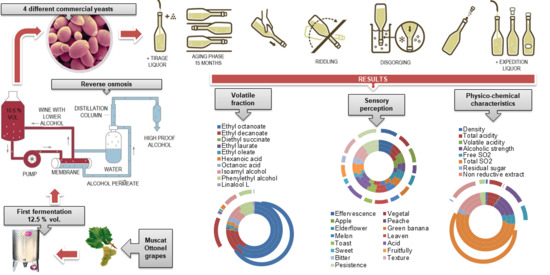
2.1. Grapes and Wine-Making Procedure
2.2. Chemicals
2.3. Methods of Analysis
3. Results and Discussion
3.1. Physical–Chemical Characteristics
3.2. Volatile Fraction
4. Sensory Characteristics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, J.E.; Kerslake, F.L.; Close, D.C.; Dambergs, R.G. Viticulture for Sparkling Wine Production: A Review. Am. J. Enol. Vitic. 2014, 65, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Koufos, G.; Mavromatis, T.; Koundouras, S.; Fyllas, N.M.; Jones, G.V. Viticulture-climate relationships in Greece: The impacts of recent climate trends on harvest date variation. Int. J. Clim. 2013, 34, 1445–1459. [Google Scholar] [CrossRef]
- Labanda, J.; Vichi, S.; Llorens, J.; López-Tamames, E. Membrane separation technology for the reduction of alcoholic degree of a white model wine. LWT 2009, 42, 1390–1395. [Google Scholar] [CrossRef]
- Saliba, A.; Ovington, L.A.; Moran, C. Consumer demand for low-alcohol wine in an Australian sample. Int. J. Wine Res. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Pickering, G.J.; Heatherbell, D.A.; Vanhanen, L.P.; Barnes, M. The effect of ethanol concentration on the tem-poral perception of viscosity and density in white wine. Am. J. Enol. Vitic. 1998, 49, 306–318. [Google Scholar]
- Takacs, L.; Vatai, G.; Korány, K. Production of alcohol free wine by pervaporation. J. Food Eng. 2007, 78, 118–125. [Google Scholar] [CrossRef]
- López, M.; Alvarez, S.; Riera, F.A.; Alvarez, R.; Álvarez-Blanco, S. Production of Low Alcohol Content Apple Cider by Reverse Osmosis. Ind. Eng. Chem. Res. 2002, 41, 6600–6606. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.; Torley, P.; Blackmann, J.W.; Scmidtke, L.M. Review of processing technology to reduce alcohol levels in wines. In Proceedings of the 1st nternational Symposium Oenoviti International, Bordeaux, France, 6 September 2013. [Google Scholar]
- Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Puertas, B.; Cantos-Villar, E.; Moreno-Rojas, J.M. Multivariate optimization of headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry for the analysis of terpenoids in sparkling wines. Talanta 2020, 208, 120483. [Google Scholar] [CrossRef]
- Coelho, E.; Coimbra, M.A.; Nogueira, J.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, A.J.; Pueyo, E. SparklingWines and Yeast Autolysis. In Wine Chemistry and Biochemistry; Springer Nature: Berlin, Germany, 2008; pp. 61–80. [Google Scholar]
- Torrens, J.; Urpí, P.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef]
- Carpentieri, A.; Sebastianelli, A.; Melchiorre, C.; Pinto, G.; Trifuoggi, M.; Lettera, V.; Amoresano, A. Fiano, Greco and Falanghina grape cultivars differentiation by volatiles fingerprinting, a case study. Heliyon 2019, 5, e02287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase micro-extraction method for headspace analysis of wine aroma components. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar]
- Sánchez-Palomo, E.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC–MS. Talanta 2005, 66, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.D.S.; De Souza, J.F.; Lima, M.D.S.; Pereira, G.E. Volatile Profiles of Sparkling Wines Produced by the Traditional Method from a Semi-Arid Region. Beverages 2018, 4, 103. [Google Scholar] [CrossRef] [Green Version]
- Escudero, A.; Charpentier, M.; Etiévant, P. Characterization of aged champagne wine aroma by GC-O and descriptive profile analyses. Sci. des Aliment. 2000, 20, 331–346. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Boschfuste, J.; Riuaumatell, M.; Guadayol, J.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography–mass spectrometry (GC–MS) analysis. Food Chem. 2007, 105, 428–435. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G.; et al. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodriguez, A.; Carrascosa, A.; Polo, M. Release of nitrogen compounds to the extracellular medium by three strains of Saccharomyces cerevisiae during induced autolysis in a model wine system. Int. J. Food Microbiol. 2001, 68, 155–160. [Google Scholar] [CrossRef]
- Vigentini, I.; Cardenas, S.B.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of Native Yeast Strains for In-Bottle Fermentation to Face the Uniformity in Sparkling Wine Production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Penacho, V.; Valero, E.; Gonzalez, R. Transcription profiling of sparkling wine second fermentation. Int. J. Food Microbiol. 2012, 153, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Pollon, M.; Rantsiou, K.; Ortiz-Julien, A.; Botto, R.; Segade, S.R.; Giacosa, S.; Rolle, L.; Cocolin, L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019, 122, 392–401. [Google Scholar] [CrossRef]
- Lencioni, L.; Romani, C.; Gobbi, M.; Comitini, F.; Ciani, M.; Domizio, P. Controlled mixed fermentation at winery scale using Zygotorulaspora florentina and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2016, 234, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Vararu, F.; Moreno-García, J.; Zamfir, C.-I.; Cotea, V.V.; Moreno, J. Selection of aroma compounds for the differentiation of wines obtained by fermenting musts with starter cultures of commercial yeast strains. Food Chem. 2016, 197, 373–381. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Vines and Musts; OIV: Paris, France, 2020. [Google Scholar]
- Benucci, I.; Esti, M. Novel microencapsulated yeast for the production of sparkling wine by traditional method. Int. J. Vitic. Enol. 2020. [Google Scholar]
- Caliari, V.; Burin, V.M.; Rosier, J.P.; BordignonLuiz, M.T. Aromatic profile of Brazilian sparkling wines produced with classical and innovative grape varieties. Food Res. Int. 2014, 62, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Caliari, V.; Panceri, C.P.; Rosier, J.P.; Bordignon-Luiz, M.T. Effect of the Traditional, Charmat and Asti method production on the volatile composition of Moscato Giallo sparkling wines. LWT 2015, 61, 393–400. [Google Scholar] [CrossRef]
- Jackson, R.S. Chemical Constituents of Grapes and Wine. Wine Sci. 2014, 347–426. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Torchio, F.; Segade, S.R.; Gerbi, V.; Cagnasso, E.; Giordano, M.; Giacosa, S.; Rolle, L. Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines. Food Res. Int. 2012, 48, 491–498. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Bosch-Fusté, J.; López-Tamames, E.; Buxaderas, S. Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem. 2006, 95, 237–242. [Google Scholar] [CrossRef]
- Delfini, C.; Formica, J. Wine Microbiology: Science and Technology (Food Science and Technology), 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ribéreau-Gayon, J. Sciences et Techniques du Vin; Dunod: Paris, France, 1972. [Google Scholar]
- Călugăr, A.; Coldea, T.E.; Pop, C.R.; Pop, T.I.; Babeș, A.C.; Bunea, C.I.; Manolache, M.; Gál, E. Evaluation of Volatile Compounds during Ageing with Oak Chips and Oak Barrel of Muscat Ottonel Wine. Processes 2020, 8, 1000. [Google Scholar] [CrossRef]
- Bidan, P. Relation entre la teneur des vins en alcools supérieurs et la teneur des moûts en substances azotées, en particulier les acides aminés. O.I.V. Bull. 1975, 536, 841–867. [Google Scholar]
- Nykänen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 1986, 37, 84–96. [Google Scholar]
- Etievant, P. Wine. Volatile Compounds in Food and Beverages; Routledge: Abingdon, UK, 1991. [Google Scholar]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Berlin, Germany, 2009; p. 746. [Google Scholar]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of croatian commer-cial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.M.; Boido, E.; Dellacassa, E. Terpenoids in Grapes and Wines: Origin and Micrometabolism during the Vinification Process. Nat. Prod. Commun. 2008, 3, 577–593. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, E. Primary aromatic character of wine. Acta Univ. Cibiniensis Ser. E Food Technol. 2012, XVI, 1. [Google Scholar]
- Heroiu, E. Research on Organic Constituents of the Aroma of Wines from the Main Varieties Cultivated in the Vineyard of Ştefăneşti-Argeş. Ph.D. Thesis, University of Bucharest, Bucharest, Romania, 1998. [Google Scholar]
- Strauss, C.R.; Wilson, B.; Gooley, P.R.; Williams, P.J. Role of Monoterpenes in Grape and Wine Flavor. ACS Symp. Ser. 1986, 222–242. [Google Scholar] [CrossRef]
- Marcon, Â.R.; Schwarz, L.; Dutra, S.; Delamare, A.; Gottardi, F.; Parpinello, G.; Echeverrigaray, S. Chemical Composition and Sensory Evaluation of Wines Produced with Different Moscato Varieties; EDP Sciences: Les Ulis, France, 2019; Volume 12, p. 02033. [Google Scholar]
- Lanaridis, P.; Salaha, M.-J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile compounds in grapes and wines from two Muscat varieties cultivated in Greek islands. OENO One 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Torrens, J.; Riu-Aumatell, M.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Assessment of Volatile and Sensory Profiles between Base and Sparkling Wines. J. Agric. Food Chem. 2010, 58, 2455–2461. [Google Scholar] [CrossRef]
- Hui, Y.H.; Chen, F.; Nollet, L.M.L.; Guiné, R.P.F.; Martín-Belloso, O.; Mínguez-Mosquera, I.M.; Paliyath, G.; Pessoa, F.L.P.; Quéré, L.J.; Sidhu, J.S.; et al. Handbook of Fruit and Vegetable Flavors, 1st ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Antonelli, A.; Castellari, L.; Zambonelli, C.; Carnacini, A. Yeast Influence on Volatile Composition of Wines. J. Agric. Food Chem. 1999, 47, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Ivit, N.N.; Kemp, B. The Impact of Non-Saccharomyces Yeast on Traditional Method Sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin, Germany, 2007. [Google Scholar]





| Samples | ρ | T. A. (g tartaric acid L−1) | V. A. (g acetic acid L−1) | A. S. (% vol.) | Free SO2 (mg L−1) | Total SO2 (mg L−1) | R. S. (g L−1) | N. E. (g L−1) | pH |
|---|---|---|---|---|---|---|---|---|---|
| V0 | 0.9932 ± 0.0001 | 6.3 ± 0.07 | 0.30 ± 0.02 | 12.5 ± 0.03 | 18 ± 0.47 | 72 ± 0.47 | 3.4 ± 0.13 | 17.0 ± 0.14 | 2.9 ± 0.02 |
| V0’ | 0.9921 ± 0.0001 | 6.2 ± 0.03 | 0.30 ± 0.01 | 10.5 ± 0.05 | 17 ± 0.47 | 71 ± 0.00 | 3.2 ± 0.05 | 15.2 ± 0.13 | 2.8 ± 0.05 |
| V1 | 0.9905 ± 0.0003 | 6.7 ± 0.02 | 0.30 ± 0.01 | 11.6 ± 0.07 | 5 ± 0.00 | 56 ± 0.47 | 0.7 ± 0.02 | 14.5 ± 0.04 | 3.1 ± 0.01 |
| V2 | 0.9908 ± 0.0001 | 6.9 ± 0.01 | 0.35 ± 0.01 | 11.3 ± 0.00 | 5 ± 0.00 | 49 ± 0.47 | 1.9 ± 0.01 | 13.3 ± 0.05 | 3.0 ± 0.01 |
| V3 | 0.9906 ± 0.0001 | 6.9 ± 0.03 | 0.30 ± 0.05 | 11.6 ± 0.00 | 5 ± 0.47 | 51 ± 0.47 | 1.9 ± 0.01 | 13.5 ± 0.01 | 3.0 ± 0.01 |
| V4 | 0.9907 ± 0.0002 | 6.6 ± 0.01 | 0.30 ± 0.09 | 11.3 ± 0.07 | 8 ± 0.47 | 64 ± 0.00 | 0.7 ± 0.02 | 14.3 ± 0.01 | 3.0 ± 0.01 |
| p-value | 0.0001 | 0.0001 | 0.0772 | 0.0772 | 0.0772 | 0.0772 | 0.0772 | 0.0772 | 0.0772 |
| No | V. C. (μg L−1) | V1 | V2 | V3 | V4 | Odor Descriptors | References |
|---|---|---|---|---|---|---|---|
| ESTERS | |||||||
| 1 | Isoamyl acetate | 17.83 ± 0.06 * | 11.71 ± 0.15 * | 17.89 ± 0.23 * | 22.78 ± 0.11 * | fruity, banana | [20] |
| 2 | Ethyl octanoate | 7998.72 ± 0.15 * | 5285.90 ± 0.08 * | 7162.47 ± 0.21 * | 6789.59 ± 0.31 * | fruity, banana, apple, pineapple, pears, floral, sweet, soap | [7,50] |
| 3 | Ethyl decanoate | 2177.35 ± 0.35 * | 985.37 ± 0.20 * | 2126.20 ± 0.11 * | 1593.61 ± 0.30 * | fruity, apple, waxy, oily | [50] |
| 4 | Diethyl succinate | 53.58 ± 0.90 * | 62.58 ± 0.01 * | 49.52 ± 0.11 * | 54.40 ± 0.57 * | fruity, floral, waxy, dusty | [7] |
| 5 | 2-Phenethyl acetate | 34.87 ± 1.57 * | 22.33 ± 0.81 * | 47.72 ± 0.44 * | 28.18 ± 0.16 * | floral, sweet, fruity, honey | [13] |
| 6 | Ethyl laurate | 162.34 ± 0.51 * | 56.71 ± 0.01 * | 136.25 ± 0.45 * | 110.49 ± 0.82 * | floral, fruity, grassy, woody | [27,50] |
| 7 | Isopropyl myristate | 14.87 ± 0.17 * | 16.17 ± 0.50 * | 15.99 ± 0.11 * | 13.98 ± 0.76 * | faint, oily, fatty | [27] |
| 8 | Ethyl palmitate | 15.64 ± 0.98 * | 7.89 ± 0.15 * | 15.98 ± 0.15 * | 8.51 ± 0.22 * | waxy, fruity, creamy and milky with a vanilla balsamic nuance | [13] |
| 9 | Ethyl oleate | 159.21 ± 0.08 * | 132.12 ± 0.16 * | 198.97 ± 0.19 * | 108.42 ± 0.23 * | fatty, oily, dairy, milky, waxy, tallow | [13] |
| ACIDS | |||||||
| 10 | Butyric acid | nd | 9.81 ± 0.57 * | nd | 6.32 ± 0.11 * | cheese, rancid, sweet, animal | [7,50] |
| 11 | Hexanoic acid | 326.09 ± 0.25 * | 189.98 ± 0.11 * | 227.50 ± 0.70 * | 230.34 ± 0.45 * | fatty | [13,51] |
| 12 | Octanoic acid | 580.64 ± 3.22 * | 258.79 ± 2.23 * | 367.50 ± 0.40 * | nd | cheese | [39] |
| 13 | Decanoic acid | 145.25 ± 0.59 * | 11.36 ± 0.06 | 13.01 ± 0.06 * | 16.91 ± 0.14 * | rancid, sour, oily, unpleasant, woody | [50,52] |
| 14 | 9-Decenoic acid | 6.90 ± 2.25 | 6.75 ± 0.98 * | 4.37 ± 1.30 | 4.00 ± 2.20 | waxy orange, reminiscent of kiwifruit, fruity and milky, melon note | [27] |
| ALCOHOLS | |||||||
| 15 | Isoamyl alcohol | 1001.47 ± 0.23 * | 485.91 ± 0.16 * | 1019.50 ± 0.02 * | 693.63 ± 0.50 * | alcohol, nail polish, bananas | [16,50] |
| 16 | 4-Octanol | 5.62 ± 0.59 | 5.53+0.75 | 6.13 ± 0.40 | 5.14 ± 0.56 | - | - |
| 17 | 1-Heptanol | 5.89 ± 0.54 * | 28.70 ± 0.04 * | 10.06 ± 0.40 * | 18.76 ± 0.45 * | musty, violet, herbal, woody, peony | [50] |
| 18 | Phenylethyl alcohol | 1150.12 ± 0.23 * | 884.56 ± 0.14 * | 973.18 ± 0.03 * | 683.46 ± 0.01 * | floral, rose, dried rose | [50] |
| TERPENIC COMPOUNDS | |||||||
| 19 | Linalool L | 138.86 ± 0.06 * | 16.65 ± 0.55 * | 120.43 ± 0.01 * | 44.31 ± 2.22 * | citrus, floral, bois de rose, green blueberry | [50] |
| 20 | α-terpineol | 42.79 ± 0.40 * | 28.19 ± 0.14 * | 41.40 ± 0.02 * | 24.19 ± 0.85 * | pine like, lilac, citrus, woody, floral | [27] |
| Variables | Groups | Diff | p | 95% Confidence Interval for Mean | |
|---|---|---|---|---|---|
| Lower Bond | Upper Bond | ||||
| Diethyl succinate | V3-V4 | 4.8800 | 0.0000 | 3.7558 | 6.0042 |
| Butyric acid | V2-V4 | −3.4900 | 0.0000 | −4.0993 | −2.8807 |
| Decanoic acid | V2-V3 | 1.6500 | 0.0000 | 1.0073 | 2.2927 |
| V3-V4 | 3.9000 | 0.0021 | 3.2573 | 4.5427 | |
| 1-Heptanol | V1-V3 | 4.1700 | 0.0000 | 3.3200 | 5.0200 |
| Linalool L | V2-V3 | −4.220 | 0.0011 | −6.6216 | −1.8184 |
| α-terpineol | V1-V3 | −1.3900 | 0.0065 | −2.3872 | −0.3928 |
| V2-V4 | −4.0000 | 0.0000 | −4.9972 | −3.0028 | |
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| Eigenvalue | 11.964 | 5.081 | 2.955 |
| Variability (%) | 59.820 | 25.404 | 14.775 |
| Cumulative % | 59.820 | 85.225 | 100.000 |
| Isoamyl acetate | 0.264 | −0.915 | −0.305 |
| Ethyl octanoate | 0.924 | −0.317 | −0.211 |
| Ethyl decanoate | 0.944 | −0.319 | 0.089 |
| Diethyl succinate | −0.219 | 0.941 | 0.257 |
| 2-Phenethyl acetate | 0.727 | −0.308 | 0.613 |
| Ethyl laurate | 0.951 | −0.287 | −0.118 |
| Isopropyl myristate | 0.234 | 0.844 | 0.483 |
| Ethyl palmitate | −0.261 | −0.413 | 0.873 |
| Ethyl oleate | 0.664 | 0.231 | 0.711 |
| Butyric acid | −0.964 | 0.186 | −0.192 |
| Hexanoic acid | 0.857 | 0.057 | −0.512 |
| Octanoic acid | 0.788 | 0.611 | 0.075 |
| Decanoic acid | 0.753 | 0.326 | −0.571 |
| 9-Decenoic acid | 0.687 | 0.651 | −0.321 |
| Isoamyl alcohol | 0.952 | −0.232 | 0.202 |
| 4-Octanol | 0.832 | −0.516 | 0.203 |
| 1-Heptanol | −0.973 | 0.228 | 0.012 |
| Phenylethyl alcohol | 0.796 | 0.603 | 0.060 |
| Linalool L | 0.992 | −0.044 | 0.117 |
| α-terpineol | 0.786 | 0.602 | −0.139 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotea, V.V.; Focea, M.C.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Marius, N.; Zamfir, C.I.; Popîrdă, A. Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines. Foods 2021, 10, 247. https://doi.org/10.3390/foods10020247
Cotea VV, Focea MC, Luchian CE, Colibaba LC, Scutarașu EC, Marius N, Zamfir CI, Popîrdă A. Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines. Foods. 2021; 10(2):247. https://doi.org/10.3390/foods10020247
Chicago/Turabian StyleCotea, Valeriu V., Mihai Cristian Focea, Camelia Elena Luchian, Lucia Cintia Colibaba, Elena Cristina Scutarașu, Niculaua Marius, Cătălin Ioan Zamfir, and Andreea Popîrdă. 2021. "Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines" Foods 10, no. 2: 247. https://doi.org/10.3390/foods10020247
APA StyleCotea, V. V., Focea, M. C., Luchian, C. E., Colibaba, L. C., Scutarașu, E. C., Marius, N., Zamfir, C. I., & Popîrdă, A. (2021). Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines. Foods, 10(2), 247. https://doi.org/10.3390/foods10020247