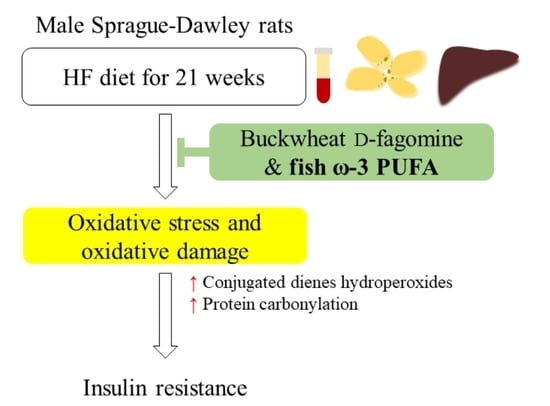
The Effects of the Combination of Buckwheat D-Fagomine and Fish Omega-3 Fatty Acids on Oxidative Stress and Related Risk Factors in Pre-Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Measurement of Glucose Tolerance
2.4. Sample Processing
2.5. Measurements of Insulin Resistance Biomarkers
2.6. Measurements of Lipid Profile, Transaminases and Interleukin-6
2.7. Measurements of Oxidative Stress Biomarkers
2.7.1. Plasma Non-Enzymatic Antioxidant Capacity
2.7.2. Antioxidant Enzymes and Glutathione
2.7.3. Lipid Peroxidation and Protein Carbonylation
2.8. Statistical Analysis
3. Results
3.1. Feed Intake, Biometric Data and Insulin Resistance Biomarkers
3.2. Lipid Profile, Transaminases and Interleukin-6
3.3. Oxidative Stress Biomarkers in Blood
3.4. Oxidative Stress Biomarkers in Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial Fatty Acid Oxidation in Obesity. Antioxid. Redox Signal. 2013, 19, 269–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Després, J.-P.; Lemieu, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Ng, M. Global, regional and national prevalence of overweight and obesity in children and adults 1980–2013: A systematic analysis. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary patterns and metabolic syndrome in adult subjects: A systematic review and meta-analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.-I.; et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008, 57, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Taltavull, N.; Romeu, M.; Amézqueta, S.; Dasilva, G.; Medina, I.; Torres, J.L. Functional Effects of the Buckwheat Iminosugar d-Fagomine on Rats with Diet-Induced Prediabetes. Mol. Nutr. Food Res. 2018, 62, e1800373. [Google Scholar] [CrossRef]
- Taltavull, N.; Ras, R.; Mariné, S.; Romeu, M.; Giralt, M.; Méndez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L.; Nogués, M.R. Protective effects of fish oil on pre-diabetes: A lipidomic analysis of liver ceramides in rats. Food Funct. 2016, 7, 3981–3988. [Google Scholar] [CrossRef]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Romeu, M.; Giralt, M.; Taltavull, N.; Muñoz-Cortes, M.; Jáuregui, O.; Méndez, L.; Medina, I.; et al. Effect of n-3 PUFA supplementation at different EPA:DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. Br. J. Nutr. 2015, 113, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Lluís, L.; Taltavull, N.; Muñoz-Cortés, M.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Molinar-Toribio, E.; Torres, J.L.; Pérez-Jiménez, J.; Pazos, M.; et al. Protective effect of the omega-3 polyunsaturated fatty acids: Eicosapentaenoic acid/Docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis. 2013, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Gómez, L.; Taltavull, N.; Nogués, M.R.; Adeva, A.; Jáuregui, O.; Joglar, J.; Clapés, P.; et al. D-Fagomine attenuates metabolic alterations induced by a high-energy-dense diet in rats. Food Funct. 2015, 6, 2614–2619. [Google Scholar] [CrossRef]
- Amézqueta, S.; Galán, E.; Vila-Fernández, I.; Pumarola, S.; Carrascal, M.; Abian, J.; Ribas-Barba, L.; Serra-Majem, L.; Torres, J.L. The presence of D-fagomine in the human diet from buckwheat-based foodstuffs. Food Chem. 2013, 136, 1316–1321. [Google Scholar] [CrossRef]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.Á.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. D-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef] [Green Version]
- Taltavull, N.; Muñoz-Cortés, M.; Lluís, L.; Jové, M.; Fortuño, À.; Molinar-Toribio, E.; Torres, J.L.; Pazos, M.; Medina, I.; Nogués, M.R. Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome. Lipids Health Dis. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Ramos-Romero, S.; Torres, J.L.; Romeu, M.; Nogués, M.R.; Medina, I. A lipidomic study on the regulation of inflammation and oxidative stress targeted by marine ω-3 PUFA and polyphenols in high-fat high-sucrose diets. J. Nutr. Biochem. 2017, 43, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined d-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.J. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies: II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Muñoz, S.; Méndez, L.; Dasilva, G.; Torres, J.L.; Ramos-Romero, S.; Romeu, M.; Nogués, M.R.; Medina, I. Targeting hepatic protein carbonylation and oxidative stress occurring on diet-induced metabolic diseases through the supplementation with fish oils. Mar. Drugs 2018, 16, 353. [Google Scholar] [CrossRef] [Green Version]
- Méndez, L.; Pazos, M.; Molinar-Toribio, E.; Sánchez-Martos, V.; Gallardo, J.M.; Rosa Nogués, M.; Torres, J.L.; Medina, I. Protein carbonylation associated to high-fat, high-sucrose diet and its metabolic effects. J. Nutr. Biochem. 2014, 25, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Feillet-Coudray, C.; Aoun, M.; Fouret, G.; Bonafos, B.; Ramos, J.; Casas, F.; Cristol, J.P.; Coudray, C. Effects of long-term administration of saturated and n-3 fatty acid-rich diets on lipid utilisation and oxidative stress in rat liver and muscle tissues. Br. J. Nutr. 2013, 110, 1789–1802. [Google Scholar] [CrossRef] [Green Version]
- Ciapaite, J.; Van Den Broek, N.M.; Te Brinke, H.; Nicolay, K.; Jeneson, J.A.; Houten, S.M.; Prompers, J.J. Differential effects of short- and long-term high-fat diet feeding on hepatic fatty acid metabolism in rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Molinar-Toribio, E.; Pérez-Jiménez, J.; Taltavull, N.; Dasilva, G.; Romeu, M.; Medina, I.; Torres, J.L. The combined action of omega-3 polyunsaturated fatty acids and grape proanthocyanidins on a rat model of diet-induced metabolic alterations. Food Funct. 2016, 7, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Selenscig, D.; Ferreira, M.D.R.; Chicco, A.; Lombardo, Y.B. Dietary fish oil ameliorates adipose tissue dysfunction in insulin-resistant rats fed a sucrose-rich diet improving oxidative stress, peroxisome proliferator-activated receptor γ and uncoupling protein 2. Food Funct. 2018, 9, 2496–2507. [Google Scholar] [CrossRef]
- Valenzuela, R.; Espinosa, A.; González-Mañán, D.; D’Espessailles, A.; Fernández, V.; Videla, L.A.; Tapia, G. N-3 Long-Chain Polyunsaturated Fatty Acid Supplementation Significantly Reduces Liver Oxidative Stress in High Fat Induced Steatosis. PLoS ONE 2012, 7, e46400. [Google Scholar] [CrossRef] [Green Version]
- Neschen, S.; Morino, K.; Dong, J.; Wang-Fischer, Y.; Cline, G.W.; Romanelli, A.J.; Rossbacher, J.C.; Moore, I.K.; Regittnig, W.; Munoz, D.S.; et al. N-3 Fatty Acids Preserve Insulin Sensitivity in Vivo in a Peroxisome Proliferator-Activated Receptor-A-Dependent Manner. Diabetes 2007, 56, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Méndez, L.; Ciordia, S.; Fernández, M.S.; Juárez, S.; Ramos, A.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Nogués, M.R.; Medina, I. Changes in liver proteins of rats fed standard and high-fat and sucrose diets induced by fish omega-3 PUFAs and their combination with grape polyphenols according to quantitative proteomics. J. Nutr. Biochem. 2017, 41, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Gianotti, T.F.; Dopazo, H.; Rohr, C.; Gaj, G.; Martino, J.S.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Ruhl, C.E.; Everhart, J.E. The association of low serum alanine aminotransferase activity with mortality in the us population. Am. J. Epidemiol. 2013, 178, 1702–1711. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Chiu, C.Y.; Wang, L.P.; Chiang, M.T. Omega-3 fatty acids-enriched fish oil activates AMPK/PGC-1α signaling and prevents obesity-related skeletal muscle wasting. Mar. Drugs 2019, 17, 380. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feillet-Coudray, C.; Fouret, G.; Vigor, C.; Bonafos, B.; Jover, B.; Blachnio-Zabielska, A.; Rieusset, J.; Casas, F.; Gaillet, S.; Landrier, J.F.; et al. Long-Term Measures of Dyslipidemia, Inflammation, and Oxidative Stress in Rats Fed a High-Fat/High-Fructose Diet. Lipids 2019, 54, 81–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G. Inflammation and metabolic disorders. Nature 2006, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Cholesterol versus inflammation as cause of chronic diseases. Nutrients 2019, 11, 2332. [Google Scholar] [CrossRef] [Green Version]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J.; Kudova, E.; et al. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAS) with anti-inflammatory properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depner, C.M.; Traber, M.G.; Bobe, G.; Kensicki, E.; Bohren, K.M.; Milne, G.; Jump, D.B. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in LDLR-/- mice. PLoS ONE 2013, 8, e83756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Vanden Heuvel, J.P.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005, 336, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ellis, J.M.; Wolfgang, M.J. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015, 10, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Veskoukis, A.S.; Nikolaidis, M.G.; Kyparos, A.; Kouretas, D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009, 47, 1371–1374. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, F.; Morino, K.; Ugi, S.; Ishikado, A.; Kondo, K.; Sato, D.; Konno, S.; Nemoto, K.I.; Kusunoki, C.; Sekine, O.; et al. 4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem. Biophys. Res. Commun. 2014, 443, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feng, X.; Zhang, J.; Zhang, Y.; Xia, F.; Liu, L.; Jin, Z.; Lu, C.; Xia, Y.; Papadimos, T.J.; et al. Ω-3 Fish Oil Fat Emulsion Preconditioning Mitigates Myocardial Oxidative Damage in Rats Through Aldehydes Stress. Biomed. Pharmacother. 2019, 118, 109198. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, B.B.; Han, L.; Gao, C.F.; Wang, M. D-Fagomine Attenuates High Glucose-Induced Endothelial Cell Oxidative Damage by Upregulating the Expression of PGC-1α. J. Agric. Food Chem. 2018, 66, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
| STD | HF | FG | ω-3 | FG&ω-3 | |
|---|---|---|---|---|---|
| Plasma lipid profile | |||||
| TG (mmol/L) | 0.7 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.1 |
| TC (mmol/L) | 3.6 ± 0.4 | 2.9 ± 0.5 a | 3.1 ± 0.5 | 2.5 ± 0.4 a | 2.6 ± 0.3 a |
| HDL (mmol/L) | 1.15 ± 0.12 | 0.94 ± 0.16 a | 0.94 ± 0.07 a | 0.85 ± 0.12 a | 0.93 ± 0.07 a |
| LDL (mmol/L) | 0.43 ± 0.11 | 0.39 ± 0.09 | 0.47 ± 0.11 | 0.32 ± 0.07 c | 0.36 ± 0.03 |
| LDL/HDL ratio | 0.37 ± 0.08 | 0.42 ± 0.06 | 0.50 ± 0.11 a | 0.38 ± 0.07 c | 0.37 ± 0.04 c |
| Liver lipid profile | |||||
| TG (µmol/g tissue) | 17.6 ± 1.0 | 16.6 ± 2.6 | 14.6 ± 1.3 | 14.1 ± 2.3 a | 16.7 ± 2.5 |
| TC (µmol/g tissue) | 5.9 ± 0.7 | 7.8 ± 1.8 a | 7.8 ± 0.8 a | 6.4 ± 1.0 | 7.6 ± 1.0 a |
| Transaminases and IL-6 | |||||
| Plasma AST (U/L) | 70.2 ± 21.9 | 57.1 ± 10.7 | 49.2 ± 8.4 | 53.6 ± 10.1 | 61.0 ± 14.4 |
| Plasma ALT (U/L) | 27.1 ± 8.2 | 19.0 ± 3.9 a | 20.2 ± 4.7 | 22.8 ± 4.2 | 24.0 ± 3.6 b,c |
| Plasma AST/ALT ratio | 2.64 ± 0.52 | 3.14 ± 0.92 | 2.49 ± 0.45 | 2.37 ± 0.29 | 2.53 ± 0.38 |
| Plasma IL-6 (pg/mL) | 522 ± 307 | 283 ± 167 | 452 ± 259 | 349 ± 184 | 154 ± 72 a |
| STD | HF | FG | ω-3 | FG&ω-3 | |
|---|---|---|---|---|---|
| Plasma | |||||
| ORAC (µmol trolox Eq/mL) | 18.3 ± 3.8 | 17.4 ± 4.7 | 18.4 ± 5.7 | 21.2 ± 6.1 | 21.1 ± 7.6 |
| FRAP (µmol trolox Eq/mL) | 0.16 ± 0.03 | 0.14 ± 0.03 | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.03 |
| GSH (nmol/mL) | 9.9 ± 1.4 | 11.2 ± 3.8 | 11.4 ± 6.2 | 10.4 ± 1.9 | 9.9 ± 3.2 |
| GSSG (nmol/mL) | 32.4 ± 2.3 | 30.5 ± 3.3 | 29.6 ± 4.9 | 29.7 ± 4.9 | 29.3 ± 1.9 |
| GSSG/GSH ratio | 3.24 ± 0.57 | 2.96 ± 0.85 | 3.19 ± 1.57 | 2.88 ± 0.41 | 3.22 ± 1.00 |
| Conjugated dienes (mmol hydroperoxides/kg lipid) | 0.13 ± 0.03 | 0.21 ± 0.06 a | 0.17 ± 0.03 a | 0.14 ± 0.01 b | 0.14 ± 0.02 |
| Albumin carbonylation index | 0.17 ± 0.04 | 0.42 ± 0.14 a | 0.36 ± 0.08a | 0.26 ± 0.05 a,b,c | 0.31 ± 0.06 a |
| Erythrocytes | |||||
| SOD (U/g Hb) | 1155 ± 410 | 1022 ± 279 | 1156 ± 245 | 1250 ± 367 | 968 ± 289 |
| CAT (mmol/g Hb) | 77.5 ± 11.4 | 86.6 ± 22.3 | 71.4 ± 12.8 | 64.1 ± 6.9 | 70.1 ± 18.6 |
| GPx (U/g Hb) | 85.4 ± 14.7 | 83.4 ± 13.1 | 76.7 ± 13.2 | 71.3 ± 6.8 a,b | 61.3 ± 14.4 a,b,c |
| GR (U/g Hb) | 0.31 ± 0.06 | 0.24 ± 0.04 | 0.21 ± 0.07 a | 0.18 ± 0.03 a | 0.19 ± 0.05 a |
| GSH (µmol/g Hb) | 1.51 ± 0.49 | 1.16 ± 1.06 | 0.69 ± 0.22 a | 0.78 ± 0.57 a | 1.69 ± 0.53 c,d |
| GSSG (µmol/g Hb) | 0.85 ± 0.26 | 0.77 ± 0.28 | 0.82 ± 0.30 | 0.93 ± 0.44 | 0.43 ± 0.08 a,b,c,d |
| GSSG/GSH ratio | 0.60 ± 0.25 | 1.29 ± 0.99 | 1.21 ± 0.34 | 1.56 ± 0.87 | 0.29 ± 0.11 d |
| STD | HF | FG | ω-3 | FG&ω-3 | |
|---|---|---|---|---|---|
| Perigonadal white adipose tissue | |||||
| SOD (U/g tissue) | 83.9 ± 13.1 | 75.5 ± 7.0 | 80.8 ± 28.8 | 63.0 ± 18.2 | 74.7 ± 24.4 |
| CAT (mmol/g tissue) | 0.11 ± 0.01 | 0.07 ± 0.04 | 0.10 ± 0.04 | 0.10 ± 0.03 | 0.08 ± 0.06 |
| GPx (U/g tissue) | 0.25 ± 0.23 | 0.07 ± 0.06 | 0.15 ± 0.20 | 0.05 ± 0.04 | 0.05 ± 0.05 |
| GR (U/g tissue) | 0.15 ± 0.09 | 0.10 ± 0.08 | 0.14 ± 0.05 | 0.14 ± 0.07 | 0.12 ± 0.07 |
| GSH (nmol/g tissue) | 6.79 ± 1.23 | 6.71 ± 2.79 | 5.75 ± 2.84 | 10.92 ± 0.93 a,b,c | 8.24 ± 3.76 |
| GSSG (nmol/g tissue) | 28.3 ± 6.7 | 24.2 ± 6.4 | 26.2 ± 4.5 | 27.3 ± 4.3 | 23.5 ± 5.1 |
| GSSG/GSH ratio | 4.32 ± 1.49 | 3.87 ± 0.86 | 5.31 ± 1.82 b | 2.52 ± 0.54 a,b,c | 3.13 ± 0.96 c |
| Liver | |||||
| SOD (U/g tissue) | 4523 ± 271 | 2896 ± 883 a | 4122 ± 1556 | 3714 ± 917 | 2956 ± 379 a |
| CAT (mmol/g tissue) | 17.7 ± 0.9 | 15.5 ± 1.4 | 16.1 ± 1.7 | 15.9 ± 2.5 | 17.2 ± 2.4 |
| GPx (U/g tissue) | 40.7 ± 6.1 | 34.0 ± 5.2 | 37.9 ± 2.3 | 38.3 ± 8.0 | 36.3 ± 5.6 |
| GR (U/g tissue) | 7.86 ± 1.37 | 5.46 ± 1.18 | 6.05 ± 1.49 | 6.34 ± 1.34 | 7.84 ± 2.64 |
| GSH (µmol/g tissue) | 0.86 ± 0.37 | 0.47 ± 0.29 | 0.62 ± 0.31 | 0.58 ± 0.37 | 0.57 ± 0.40 |
| GSSG (µmol/g tissue) | 1.56 ± 0.26 | 1.29 ± 0.14 | 1.22 ± 0.07 | 1.21 ± 0.26 a | 1.08 ± 0.28 a |
| GSSG/GSH ratio | 2.02 ± 0.65 | 3.46 ± 1.55 | 2.45 ± 1.15 | 2.97 ± 2.04 | 2.90 ± 2.10 |
| Conjugated dienes (mmol hydroperoxides/kg lipid) | 15.8 ± 0.5 | 23.3 ± 1.1 a | 23.8 ± 1.3 a | 21.7 ± 1.1 a | 22.6 ± 1.6 a |
| Total protein carbonylation index | 0.74 ± 0.11 | 0.94 ± 0.16 a | 0.91 ± 0.16 a | 0.75 ± 0.10 b,c | 0.63 ± 0.08 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles-Pérez, B.; Nogués, M.R.; Sánchez-Martos, V.; Taltavull, N.; Méndez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L.; Romeu, M. The Effects of the Combination of Buckwheat D-Fagomine and Fish Omega-3 Fatty Acids on Oxidative Stress and Related Risk Factors in Pre-Obese Rats. Foods 2021, 10, 332. https://doi.org/10.3390/foods10020332
Miralles-Pérez B, Nogués MR, Sánchez-Martos V, Taltavull N, Méndez L, Medina I, Ramos-Romero S, Torres JL, Romeu M. The Effects of the Combination of Buckwheat D-Fagomine and Fish Omega-3 Fatty Acids on Oxidative Stress and Related Risk Factors in Pre-Obese Rats. Foods. 2021; 10(2):332. https://doi.org/10.3390/foods10020332
Chicago/Turabian StyleMiralles-Pérez, Bernat, Maria Rosa Nogués, Vanessa Sánchez-Martos, Núria Taltavull, Lucía Méndez, Isabel Medina, Sara Ramos-Romero, Josep L. Torres, and Marta Romeu. 2021. "The Effects of the Combination of Buckwheat D-Fagomine and Fish Omega-3 Fatty Acids on Oxidative Stress and Related Risk Factors in Pre-Obese Rats" Foods 10, no. 2: 332. https://doi.org/10.3390/foods10020332
APA StyleMiralles-Pérez, B., Nogués, M. R., Sánchez-Martos, V., Taltavull, N., Méndez, L., Medina, I., Ramos-Romero, S., Torres, J. L., & Romeu, M. (2021). The Effects of the Combination of Buckwheat D-Fagomine and Fish Omega-3 Fatty Acids on Oxidative Stress and Related Risk Factors in Pre-Obese Rats. Foods, 10(2), 332. https://doi.org/10.3390/foods10020332