Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin
Abstract
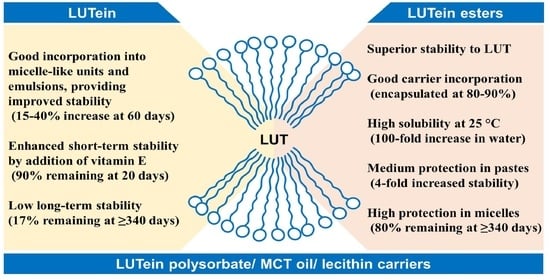
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Formulations
2.2.2. HPLC
2.2.3. Encapsulation Efficiency
2.2.4. Stability Tests
- Storage comparison: At 4 °C in the dark versus at 25 °C under a light source placed 20 cm above the sample (fluorescent light: 20 W, 6400 K, 220–240 V, 50/60 Hz; with pronounced spectral intensities at 620, 550, 440, 405 nm, in order of relative power).
- Vitamin E: Preparation of the lutein extract emulsion without (3L) and with (3L-VE) vitamin E, and comparison of storage at 25 °C in the dark.
- Accelerated storage: Simulation of accelerated conditions of storage (Incucell LSIK-B2V/IC 55 storage chamber; MMM Medcenter Einrichtungen GmbH, Germany). In the storage chamber, 1 day at 37 °C corresponded to ~4 days of storage in real time at 25 °C.
2.2.5. Colour Measurement
2.2.6. Particle Diameter and ζ-Potential
2.2.7. Laser Confocal Scanning Microscopy
3. Results and Discussion
3.1. Encapsulation Efficiency
3.2. Stability Tests
3.3. Stability of the Lutein and Lutein Esters Extracts and Their Pastes and Fine Suspensions
3.4. Stability of Lutein and Lutein Esters Syrups with Lecithin
3.5. Stability of Lutein and Lutein Esters in Syrups with Lecithin under Accelerated Conditions of Storage
3.6. Colour Stability
3.7. Physical Stability—Particle Size and ζ-Potential
3.8. Microscopy
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Subagio, A.; Wakaki, H.; Morita, N. Stability of lutein and its myristate esters. Biosci. Biotechnol. Biochem. 1999, 63, 1784–1786. [Google Scholar] [CrossRef]
- Madaan, T.; Choudhary, A.N.; Gyenwalee, S.; Thomas, S.; Mishra, H.; Tariq, M.; Vohora, D.; Talegaonkar, S. Lutein, a versatile phyto-nutraceutical: An insight on pharmacology, therapeutic indications, challenges and recent advances in drug delivery. PharmaNutrition 2017, 5, 64–75. [Google Scholar] [CrossRef]
- Bhuyian, H.U.; Islam, A.; Tareque, I.; Rashid, H.A. Development and validation of method for determination of lutein by HPLC. World J. Pharm. Res. 2015, 4, 145–156. [Google Scholar]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, H.; Jiang, P.; Yao, Y.; Han, J. Preparation of lutein-loaded particles for improving solubility and stability by Polyvinylpyrrolidone (PVP) as an emulsion-stabilizer. Food Chem. 2014, 156, 123–128. [Google Scholar] [CrossRef]
- Šivel, M.; Kráčmar, S.; Fišera, M.; Klejdus, B.; Kubáň, V. Lutein content in marigold flower (Tagetes erecta L.) concentrates used for production of food supplements. Czech. J. Food Sci. 2014, 32, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Raila, J.; Ali, M.; Islam, K.M.S.; Schenk, R.; Krause, J.P.; Schweigert, F.J.; Rawel, H. Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under food processing conditions. J. Funct. Foods 2012, 4, 602–610. [Google Scholar] [CrossRef]
- Brum, A.A.S.; dos Santos, P.P.; da Silva, M.M.; Paese, K.; Guterres, S.S.; Costa, T.M.H.; Pohlmann, A.R.; Jablonski, A.; Flôres, S.H.; de Rios, A.O. Lutein-loaded lipid-core nanocapsules: Physicochemical characterization and stability evaluation. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 522, 477–484. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef]
- Kaimainen, M.; Järvenpää, E.; Huopalahti, R. Enzyme-Assisted Oil Extraction of Lutein from Marigold (Tagetes erecta) Flowers and Stability of Lutein during Storage. Int. J. Agric. Food Res. 2015, 4, 11–19. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Health Organization Evaluation of certain food additives. World Health Organ. Tech. Rep. Ser. 2005, 928, 1–156. [Google Scholar]
- EFSA. Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbet, S.; Marquez, S.A. Complexes of lutein with bovine and caprine caseins and their impact on lutein chemical stability in emulsion systems: Effect of arabinogalactan. J. Dairy Sci. 2018, 101, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Davidov-Pardo, G.; Gumus, C.E.; Mcclements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of pH and temperature on physical and chemical stability. Food Chem. 2016, 196, 821–827. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Frede, K.; Henze, A.; Khalil, M.; Baldermann, S.; Schweigert, F.J.; Rawel, H. Stability and cellular uptake of lutein-loaded emulsions. J. Funct. Foods 2014, 8, 118–127. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of a health claim related to a combination of lutein and zeaxanthin and improved vision under bright light conditions pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3753. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Miriyala, N.; Su, Y.; Chen, W.; Gao, X.; Shao, L.; Yan, R.; Li, H.; Yao, X.; Cao, D.; et al. Computer-Aided Formulation Design for a Highly Soluble Lutein-Cyclodextrin Multiple-Component Delivery System. Mol. Pharm. 2018, 15, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, H.; Zhou, C.; Lv, F.; Bie, X.; Lu, Z. Study on the spray-drying encapsulation of lutein in the porous starch and gelatin mixture. Eur. Food Res. Technol. 2012, 234, 157–163. [Google Scholar] [CrossRef]
- Chen, H.; Guan, Y.; Zhong, Q. Microemulsions based on a sunflower lecithin-tween 20 blend have high capacity for dissolving peppermint oil and stabilizing coenzyme Q10. J. Agric. Food Chem. 2015, 63, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.S.L.; Griffin, C.; Roos, Y.H. Stability and loss kinetics of lutein and β-carotene encapsulated in freeze-dried emulsions with layered interface and trehalose as glass former. Food Res. Int. 2014, 62, 403–409. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Yokoyama, W.; Zhang, Y.; Zhao, L. Characterization of milk proteins-lutein complexes and the impact on lutein chemical stability. Food Chem. 2016, 200, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Krstonošić, V.; Milanović, M.; Dokić, L. Application of different techniques in the determination of xanthan gum-SDS and xanthan gum-Tween 80 interaction. Food Hydrocoll. 2019, 87, 108–118. [Google Scholar] [CrossRef]
- Behnam, D. Water Free Ubiquinone Concentrate. U.S. Patent 7094804 B2, 22 August 2006. [Google Scholar]
- Li, J.; Guo, R.; Hu, H.; Wu, X.; Ai, L.; Wu, Y. Preparation optimisation and storage stability of nanoemulsion-based lutein delivery systems. J. Microencapsul. 2018, 35, 570–583. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.Y.; Choi, S.J. Influence of Oxidants on the Stability of Tocopherol in Model Nanoemulsions: Role of Interfacial Membrane Organized by Nonionic Emulsifiers. J. Chem. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification Does Not Impair Lutein Bioavailability in Humans. J. Nutr. 2002, 132, 3668–3673. [Google Scholar] [CrossRef] [Green Version]
- Roodenburg, A.J.C.; Leenen, R.; Van Het Hof, K.H.; Weststrate, J.A.; Tijburg, L.B.M. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am. J. Clin. Nutr. 2000, 71, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Marais, J. 1, 1,6-Trimethyl-1,2-dihydronaphthalene (TDN): A Possible Degradation Product of Lutein and beta-Carotene. S. Afr. J. Enol. Vitic. 2017, 13, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Marais, J.; van Wyk, C.J.; Rapp, A. Effect of Storage Time, Temperature and Region on the Levels of 1, l,6-Trimethyl-1,2-dihydronaphthalene and other Volatiles, and on Quality of Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 2017, 13, 33–34. [Google Scholar] [CrossRef] [Green Version]
- Onsaard, E.; Vittayanont, M.; Srigam, S.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by coconut skim milk proteins. J. Agric. Food Chem. 2005, 53, 5747–5753. [Google Scholar] [CrossRef] [PubMed]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum Arabic, maltodextrin and inulin as coating materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Siani, A.G.; Grosso, C.R.F. Stability of monoterpenes encapsulated in gum arabic by spray-drying. J. Agric. Food Chem. 2001, 49, 780–785. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of Arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Chranioti, C.; Tzia, C. Arabic Gum Mixtures as Encapsulating Agents of Freeze-Dried Fennel Oleoresin Products. Food Bioprocess. Technol. 2014, 7, 1057–1065. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis encapsulation by spray drying: Characterization and stability. LWT Food Sci. Technol. 2017, 75, 227–235. [Google Scholar] [CrossRef]



| Formulation | Extract | Carrier | Form |
|---|---|---|---|
| 1LE | Lutein ester | Polyoxyethylene sorbitan monooleate | Paste |
| 2L, 2L-1/2/3 | Lutein | Polyoxyethylene sorbitan monooleate, medium-chain triglyceride oil | Finely dispersed suspensions |
| 2LE | Lutein ester | Polyoxyethylene sorbitan monooleate, medium-chain triglyceride oil | Finely dispersed suspensions |
| 3L | Lutein | Lecithin | Viscous emulsion |
| 3L-VE | Lutein | Lecithin, vitamin E | Viscous emulsion |
| 3LE | Lutein ester | Lecithin | Viscous emulsion |
| Condition/Formulation | Carrier | Defined Lutein/Lutein Esters:MCT Oil Mass Ratio | Calculated Lutein/Lutein Esters Content (%) | Calculated Lutein:Carrier Mass Ratio | Measured Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| Paste/ | |||||
| 1LE | PSM | / | 3.45 | 1:22.5 | 77.8 ± 0.19 |
| Fine suspension/ | |||||
| 2L | PSM | 1:3 | 3.28 | 1:22.5 | 83.1 ± 0.26 |
| 2L-1 | PSM | 1:1.5 | 2.27 | 1:37.5 | 73.5 ± 0.16 |
| 2L-2 | PSM | 1:1.6 | 3.65 | 1:23.8 | 89.9 ± 0.37 |
| 2L-3 | PSM | 1:3.2 | 3.45 | 1:23.8 | 89.2 ± 0.22 |
| 2LE | PSM | 1:1.5 | 3.45 | 1:22.5 | 79.8 ± 0.19 |
| Viscous emulsion/ | |||||
| 3L | Lecithin | / | 1.9 | 1:28.3 | 99.3 ± 0.06 |
| 3LE | Lecithin | / | 1.9 | 1:28.3 | 91.4 ± 0.01 |
| Condition/Formulation | Sample | Stabilities over Time (%) | |||
|---|---|---|---|---|---|
| 0 Days | 20 Days | 40 Days | 60 Days | ||
| Free extract/ | |||||
| - | Lutein | 100 | 45.7 ± 0.6 | 12.8 ± 0.3 | 4.8 ± 0.3 |
| - | Lutein esters | 100 | 90.5 ± 0.4 | 85.4 ± 0.3 | 64.2 ± 0.3 |
| Paste/ | |||||
| 1LE | Lutein esters | 100 | 90.5 ± 0.2 | 74.3 ± 0.3 | 68.9 ± 0.2 |
| Fine suspension/ | |||||
| 2L | Lutein | 100 | 62.7 ± 0.3 | 32.3 ± 0.6 | 20.3 ± 0.6 |
| 2LE | Lutein esters | 100 | 97.8 ± 0.5 | 82.9 ± 0.2 | 82.8 ± 0.4 |
| Viscous emulsion/ | |||||
| 3L | Lutein | 100 | 84.0 ± 0.2 | 67.8 ± 0.6 | 43.8 ± 0.5 |
| 3LE | Lutein esters | 100 | 96.7 ± 0.3 | 95.2 ± 0.2 | 93.6 ± 0.5 |
| Parameter | Stabilities over Time (%) | |||
|---|---|---|---|---|
| 0 Days | 7 Days | 21 Days | 300 Days | |
| L* | 47.3 ± 0.2 | 48.2 ± 0.2 | 45.8 ± 0.8 | 48.5 ± 0.3 |
| a* | 30.4 ± 0.5 | 30.8 ± 0.3 | 30.2 ± 0.6 | 30.2 ± 0.2 |
| b* | 42.0 ± 0.6 | 44.1 ± 0.4 | 39.3 ± 1.0 | 44.4 ± 0.2 |
| ΔE | 0 | 2.3 | 3.1 | 2.6 |
| ΔC* | 0 | 2.0 | 2.7 | 2.4 |
| Parameter | Stabilities over Time (%) | |||
|---|---|---|---|---|
| 0 Days | 7 Days | 21 Days | 300 Days | |
| L* | 46.2 ± 0.2 | 46.3 ± 0.5 | 48.1 ± 1.0 | 45.8 ± 0.4 |
| a* | 33.6 ± 0.1 | 34.3 ± 0.5 | 25.6 ± 0.4 | 33.7 ± 0.14 |
| b* | 42.7 ± 0.6 | 42.2 ± 0.6 | 43.9 ± 1.0 | 42.5 ± 0.5 |
| ΔE | 0 | 0.9 | 8.3 | 0.5 |
| ΔC* | 0 | 0.9 | 8.1 | 0.3 |
| Condition/Formulation | Parameter | Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 10 | 28 | 50 | ||
| Paste/ | |||||
| 1LE | Size (d. nm) | 848 ± 133 | 882 ± 200 | 911 ± 197 | 882 ± 205 |
| ζ-Potential (mV) | −12.4 ± 1.7 | - | - | −11.4 ± 3.8 | |
| Fine suspension/ | |||||
| 2LE | Size (d. nm) | 817 ± 195 | 722 ± 160 | 907 ± 267 | 618 ± 17 |
| ζ-Potential (mV) | −16.5 ± 3.3 | - | - | −15.0 ± 4.1 | |
| Viscous emulsion/ | |||||
| 3LE | Size (d. nm) | 380 ± 71 | 375 ± 65 | 370 ± 69 | 365 ± 56 |
| ζ-Potential (mV) | −1.5 ± 4.6 | - | - | −1.1 ± 3.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombač, Z.; Osojnik Črnivec, I.G.; Skrt, M.; Istenič, K.; Knez Knafelj, A.; Pravst, I.; Poklar Ulrih, N. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods 2021, 10, 500. https://doi.org/10.3390/foods10030500
Gombač Z, Osojnik Črnivec IG, Skrt M, Istenič K, Knez Knafelj A, Pravst I, Poklar Ulrih N. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods. 2021; 10(3):500. https://doi.org/10.3390/foods10030500
Chicago/Turabian StyleGombač, Zala, Ilja Gasan Osojnik Črnivec, Mihaela Skrt, Katja Istenič, Andreja Knez Knafelj, Igor Pravst, and Nataša Poklar Ulrih. 2021. "Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin" Foods 10, no. 3: 500. https://doi.org/10.3390/foods10030500
APA StyleGombač, Z., Osojnik Črnivec, I. G., Skrt, M., Istenič, K., Knez Knafelj, A., Pravst, I., & Poklar Ulrih, N. (2021). Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods, 10(3), 500. https://doi.org/10.3390/foods10030500