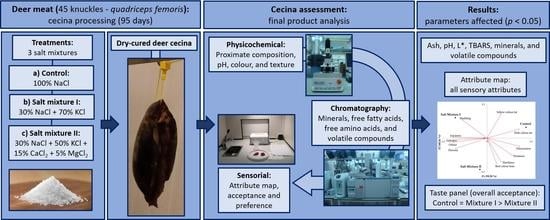
Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cecina Manufacture
2.2. Proximate Composition Analysis
2.3. Physicochemical Analysis
2.4. Free Fatty Acids Analysis
2.5. Mineral Analysis
2.6. Free Amino Acid Analysis
2.7. Volatile Compounds Analysis
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Physicochemical Parameters of Dry-Cured Deer Cecina
3.2. Mineral Composition of Dry-Cured Deer Cecina
3.3. Free Fatty Acids Content of Dry-Cured Deer Cecina
3.4. Free Amino Acids Content of Dry-Cured Deer Cecina
3.5. Volatile Compounds of Dry-Cured Deer Cecina
3.5.1. Alcohols, and Hydrocarbons
3.5.2. Ketones, Sulphur Compounds, and Esters
3.5.3. Aldehydes, Furans, Acids, Phenol, Benzene-Derived Compounds, and “Others”
3.6. Sensorial Analysis of Dry-Cured Deer Cecina
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrero, A.; Sañudo, C.; del Campo, M.M.; Olleta, J.L.; Muela, E.; Macedo, R.M.G.; Macedo, F.A.F. Consumer acceptability of dry cured meat from cull ewes reared with different linseed supplementation levels and feeding durations. Foods 2018, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Sañudo, C.; Gomes Monteiro, A.L.; Velandia Valero, M.; Fugita, C.A.; Monge, P.; Guerrero, A.; Campo, M.D.M. Cross-Cultural Study of Dry-Cured Sheep Meat Acceptability by Native and Immigrant Consumers in Spain. J. Sens. Stud. 2016, 31, 12–21. [Google Scholar] [CrossRef]
- Molinero, C.; Martínez, B.; Rubio, B.; Rovira, J.; Jaime, I. The effects of extended curing on the microbiological, physicochemical and sensorial characteristics of Cecina de León. Meat Sci. 2008, 80, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Fonseca, S.; Gómez, M.; Domínguez, R. Influence of the salting time on physico-chemical parameters, lipolysis and proteolysis of dry-cured foal “cecina". LWT Food Sci. Technol. 2015, 60, 332–338. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012; ISBN 978 92 4 150483 6. [Google Scholar]
- Vidal, V.A.S.; Bernardinelli, O.D.; Paglarini, C.S.; Sabadini, E.; Pollonio, M.A.R. Understanding the effect of different chloride salts on the water behavior in the salted meat matrix along 180 days of shelf life. Food Res. Int. 2019, 125, 108634. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldrá, F. Biochemical and sensory changes in dry-cured ham salted with partial replacements of NaCl by other chloride salts. Meat Sci. 2012, 90, 361–367. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J. Influence of anatomical retail cut on physicochemical and sensory characteristics of foal “cecina”. Int. J. Food Prop. 2016, 19, 802–813. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Campagnol, P.C.B.; Zhu, Z.; Alpas, H.; Barba, F.J.; Tomasevic, I. Technological aspects of horse meat products—A review. Food Res. Int. 2017, 102, 176–183. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Gómez, B.; Pateiro, M.; Pérez-Santaescolástica, C.; López-Fernández, O.; Sarriés, M.V.; Lorenzo, J.M. Effect of NaCl replacement by other chloride salts on physicochemical parameters, proteolysis and lipolysis of dry-cured foal “cecina”. J. Food Sci. Technol. 2020, 57, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Martínez, B.; González-Fernández, C.; Garcı´a-Cachán, M.D.; Rovira, J.; Jaime, I. Influence of storage period and packaging method on sliced dry cured beef “Cecina de Leon”: Effects on microbiological, physicochemical and sensory quality. Meat Sci. 2006, 74, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Hierro, E.; De La Hoz, L.; Ordóñez, J.A. Headspace volatile compounds from salted and occasionally smoked dried meats (cecinas) as affected by animal species. Food Chem. 2004, 85, 649–657. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Consumer perceptions towards healthier meat products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- Soriano, A.; Murillo, P.; Perales, M.; Sánchez-García, C.; Murillo, J.A.; García Ruiz, A. Nutritional quality of wild Iberian red deer (Cervus elaphus hispanicus) meat: Effects of sex and hunting period. Meat Sci. 2020, 168, 108189. [Google Scholar] [CrossRef] [PubMed]
- Kudrnáčová, E.; Bartoň, L.; Bureš, D.; Hoffman, L.C. Carcass and meat characteristics from farm-raised and wild fallow deer (Dama dama) and red deer (Cervus elaphus): A review. Meat Sci. 2018, 141, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Domínguez, R.; Pateiro, M.; Franco, D.; Barba, F.J.; Lorenzo, J.M. Chemical and physico-chemical changes during the dry-cured processing of deer loin. Int. J. Food Sci. Technol. 2020, 55, 1025–1031. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pâté. LWT 2020, 125, 109223. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Munekata, P.E.S.; Pateiro, M.; Franco, D.; Campagnol, P.C.B.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Physicochemical Composition and Nutritional Properties of Deer Burger Enhanced with Healthier Oils. Foods 2020, 9, 571. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Munekata, P.E.S.; Gagaoua, M.; Franco, D.; Campagnol, P.C.B.; Pateiro, M.; da Barretto, A.C.S.; Domínguez, R.; Lorenzo, J.M. Inclusion of Healthy Oils for Improving the Nutritional Characteristics of Dry-Fermented Deer Sausage. Foods 2020, 9, 1487. [Google Scholar] [CrossRef]
- ISO 1442. International Standards Meat and Meat Products—Determination of Moisture Content (Reference Method); International Organization for Standarization: Geneva, Switzerland, 1997. [Google Scholar]
- ISO 937. International Standards Meat and Meat Products—Determination of Nitrogen Content; International Organization for Standarization: Geneva, Switzerland, 1978. [Google Scholar]
- ISO 936. International Standards Meat and Meat Products—Determination of Total Ash; International Organization for Standarization: Geneva, Switzerland, 1998. [Google Scholar]
- AOCS. AOCS Official Procedure Am5-04. In Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- CIE. Colorimetry: Official Recommendations of the International Commission on Illumination; CIE: Vienna, Austria, 1976; No. 15 E-1.3.1. [Google Scholar]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Kaluzny, M.A.; Duncan, L.A.; Merritt, M.V.; Epps, D.E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 1985, 26, 135–140. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Domínguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- ISO 3972. Sensory Analysis—Methodology—Method of Investigating Sensitivity of Taste; International Organization for Standarization: Geneva, Switzerland, 1991. [Google Scholar]
- ISO 11036. Sensory Analysis—Methodology—Texture Profile; International Organization for Standarization: Geneva, Switzerland, 1994. [Google Scholar]
- ISO 5496. Sensory Analysis—Methodology—Initiation and Training of Assessors in the Detection and Recognition of Odours; International Organization for Standarization: Geneva, Switzerland, 2006. [Google Scholar]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldrá, F. Biochemical and sensory properties of dry-cured loins as affected by partial replacement of sodium by potassium, calcium, and magnesium. J. Agric. Food Chem. 2009, 57, 9699–9705. [Google Scholar] [CrossRef]
- Aliño, M.; Grau, R.; Toldrá, F.; Barat, J.M. Physicochemical changes in dry-cured hams salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Meat Sci. 2010, 86, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, O.; Astiasarán, I.; Bello, J. Influence of partial replacement of NaCl with KCl and CaCl2 on texture and color of dry fermented sausages. J. Agric. Food Chem. 1999, 47, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, O.; Astiasarán, I.; Bello, J. Influence of partial replacement of NaCl with KCl and CaCl2 on microbiological evolution of dry fermented sausages. Food Microbiol. 2001, 18, 329–334. [Google Scholar] [CrossRef]
- Horita, C.N.; Messias, V.C.; Morgano, M.A.; Hayakawa, F.M.; Pollonio, M.A.R. Textural, microstructural and sensory properties of reduced sodium frankfurter sausages containing mechanically deboned poultry meat and blends of chloride salts. Food Res. Int. 2014, 66, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Andrés, A.I.; Cava, R.; Ventanas, J.; Muriel, E.; Ruiz, J. Lipid oxidative changes throughout the ripening of dry-cured Iberian hams with different salt contents and processing conditions. Food Chem. 2004, 84, 375–381. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, L.; Li, J.; Tu, J.; Wang, Z. Effects of partial replacement of NaCl with KCl on bacterial communities and physicochemical characteristics of typical Chinese bacon. Food Microbiol. 2021, 93, 103605. [Google Scholar] [CrossRef]
- Jin, G.; Zhang, J.; Yu, X.; Lei, Y.; Wang, J. Crude lipoxygenase from pig muscle: Partial characterization and interactions of temperature, NaCl and pH on its activity. Meat Sci. 2011, 87, 257–263. [Google Scholar] [CrossRef]
- Blesa, E.; Aliño, M.; Barat, J.M.; Grau, R.; Toldrá, F.; Pagán, M.J. Microbiology and physico-chemical changes of dry-cured ham during the post-salting stage as affected by partial replacement of NaCl by other salts. Meat Sci. 2008, 78, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.A.S.; Santana, J.B.; Paglarini, C.S.; da Silva, M.A.A.P.; Freitas, M.Q.; Esmerino, E.A.; Cruz, A.G.; Pollonio, M.A.R. Adding lysine and yeast extract improves sensory properties of low sodium salted meat. Meat Sci. 2020, 159, 107911. [Google Scholar] [CrossRef] [PubMed]
- Contador, R.; Ortiz, A.; del Ramírez, M.R.; García-Torres, S.; López-Parra, M.M.; Tejerina, D. Physico-chemical and sensory qualities of Iberian sliced dry-cured loins from various commercial categories and the effects of the type of packaging and refrigeration time. Lwt 2021, 141, 110876. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldrá, F. Biochemical changes in dry-cured loins salted with partial replacements of NaCl by KCl. Food Chem. 2009, 117, 627–633. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Caro, I.; Blanco, C.; Morán, L.; Prieto, N.; Bodas, R.; Giráldez, F.J.; Mateo, J. Quality characteristics of a dry-cured lamb leg as affected by tumbling after dry-salting and processing time. Meat Sci. 2014, 97, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kantono, K.; Ma, Q.; Hamid, N.; Oey, I.; Farouk, M. Changes in physicochemical properties of chilled and frozen-thawed lamb cuts treated subjected to pulsed electric field processing. Food Res. Int. 2021, 141, 110092. [Google Scholar] [CrossRef]
- Luo, J.; Nasiru, M.M.; Zhuang, H.; Zhou, G.; Zhang, J. Effects of partial NaCl substitution with high-temperature ripening on proteolysis and volatile compounds during process of Chinese dry-cured lamb ham. Food Res. Int. 2021, 140, 110001. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Toldrá, F. Effect of sodium, potassium, calcium and magnesium chloride salts on porcine muscle proteases. Eur. Food Res. Technol. 2009, 229, 93–98. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Bermúdez, R.; Domínguez, R.; Guiotto, A.; Franco, D.; Purriños, L. Physicochemical and microbial changes during the manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Food Control 2015, 50, 763–769. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Tang, J.; Huang, M.; Zhao, J.; Zhang, J. Influence of partial replacement of NaCl with KCl on formation of volatile compounds in Jinhua ham during processing. Food Sci. Biotechnol. 2016, 25, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Armenteros, M.; Toldrá, F.; Aristoy, M.C.; Ventanas, J.; Estévez, M. Effect of the partial replacement of sodium chloride by other salts on the formation of volatile compounds during ripening of dry-cured ham. J. Agric. Food Chem. 2012, 60, 7607–7615. [Google Scholar] [CrossRef] [PubMed]
- Petričević, S.; Marušić Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- García-González, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Sci. 2008, 80, 315–325. [Google Scholar] [CrossRef]
- Purriños, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, C.; Sirtori, F.; Calamai, L.; Franci, O. The evolution of volatile compounds profile of “Toscano” dry-cured ham during ripening as revealed by SPME-GC-MS approach. J. Mass Spectrom. 2010, 45, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Chastagnac, C.; Vergnais, L.; Montel, M.C.; Berdagué, J.L. Production of esters by Staphylococci. Int. J. Food Microbiol. 1998, 45, 143–150. [Google Scholar] [CrossRef]
- Wang, J.; Jin, G.; Zhang, W.; Ahn, D.U.; Zhang, J. Effect of curing salt content on lipid oxidation and volatile flavour compounds of dry-cured turkey ham. LWT Food Sci. Technol. 2012, 48, 102–106. [Google Scholar] [CrossRef]
- Rodrigues, S.; Teixeira, A. Use of generalized Procrustes analysis (GPA) to test the effects of sex and carcass weight on sensory quality evaluations of Terrincho lamb meat. Meat Sci. 2013, 93, 485–488. [Google Scholar] [CrossRef] [PubMed]
- ISO 8587. Sensory Analysis—Methodology—Ranking; International Organization for Standarization: Geneva, Switzerland, 2006. [Google Scholar]


| Control | Salt Mixture I | Salt Mixture II | SEM | Sig. | |
|---|---|---|---|---|---|
| Proximate composition (g/100 g) | |||||
| Moisture | 31.98 | 30.22 | 31.60 | 0.52 | ns |
| Fat | 1.45 | 2.39 | 2.19 | 0.18 | ns |
| Protein | 56.32 | 55.99 | 55.18 | 0.45 | ns |
| Ash | 8.01 c | 9.71 a | 8.71 b | 0.15 | *** |
| pH | 5.94 b | 6.17 a | 5.83 c | 0.030 | *** |
| Colour parameters | |||||
| L* | 25.85 b | 30.71 a | 26.09 b | 0.81 | * |
| a* | 3.44 | 3.33 | 3.19 | 0.16 | ns |
| b* | 3.19 | 4.61 | 3.11 | 0.34 | ns |
| TBARS (mg MDA/kg) | 3.28 a | 2.60 b | 2.41 b | 0.14 | * |
| Texture parameters | |||||
| Firmness (N/s) | 12.57 | 10.76 | 8.19 | 0.82 | ns |
| Total work (N mm) | 311.84 | 326.59 | 268.11 | 19.14 | ns |
| Shear force (N/cm2) | 43.046 | 37.29 | 31.08 | 2.33 | ns |
| Minerals (mg/100 g) | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|
| Ca | 13.55 b | 12.08 b | 265.85 a | 18.62 | *** |
| Fe | 7.95 | 7.64 | 7.06 | 0.24 | ns |
| K | 851.66 c | 3169.45 a | 2459.89 b | 163.01 | *** |
| Mg | 46.21 b | 44.10 b | 104.09 a | 4.48 | *** |
| Mn (µg/100 g) | 39.42 | 42.56 | 46.34 | 2.09 | ns |
| Na | 1844.86 a | 759.20 b | 706.73 b | 82.30 | *** |
| P | 476.25 | 462.54 | 471.66 | 7.21 | ns |
| Free Fatty Acids (g/100 g of fat) | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|
| C14:0 | 0.42 | 0.40 | 0.36 | 0.023 | ns |
| C14:1n-5 | 0.12 | 0.13 | 0.11 | 0.0096 | ns |
| C15:0 | 0.19 a | 0.15 b | 0.14 b | 0.0061 | ** |
| C15:1n-5 | 6.21 | 5.60 | 5.60 | 0.16 | ns |
| C16:0 | 6.01 | 5.41 | 5.41 | 0.16 | ns |
| C16:1n-7 | 0.79 | 0.79 | 0.74 | 0.047 | ns |
| C17:0 | 0.31 | 0.25 | 0.26 | 0.011 | ns |
| C17:1n-7 | 0.081 | 0.071 | 0.075 | 0.0036 | ns |
| C18:0 | 5.98 | 5.23 | 5.46 | 0.16 | ns |
| 11t-C18:1 | 0.30 | 0.32 | 0.26 | 0.014 | ns |
| C18:1n-9 | 4.73 | 4.58 | 4.92 | 0.22 | ns |
| C18:1n-7 | 0.70 | 0.74 | 0.71 | 0.032 | ns |
| C18:2n-6 | 6.06 | 5.41 | 5.61 | 0.18 | ns |
| C18:3n-3 | 1.60 | 1.34 | 1.29 | 0.068 | ns |
| C20:3n-6 | 0.25 | 0.23 | 0.26 | 0.012 | ns |
| C20:4n-6 | 2.64 | 2.47 | 2.75 | 0.11 | ns |
| C20:5n-3 | 0.65 | 0.59 | 0.57 | 0.027 | ns |
| C22:5n-3 | 0.91 | 0.87 | 0.92 | 0.036 | ns |
| C22:6n-3 | 0.19 | 0.18 | 0.18 | 0.011 | ns |
| SFA | 13.05 | 11.61 | 11.78 | 0.32 | ns |
| MUFA | 12.67 | 11.94 | 12.21 | 0.41 | ns |
| PUFA | 12.47 | 11.26 | 11.75 | 0.38 | ns |
| n-6 | 9.07 | 8.22 | 8.76 | 0.28 | ns |
| n-3 | 3.35 | 2.98 | 2.95 | 0.13 | ns |
| Total free fatty acids | 38.52 | 35.15 | 36.04 | 0.91 | ns |
| Free Amino Acids (mg/100 g Dry Matter) | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|
| Aspartic acid | 6.01 | 4.62 | 5.40 | 0.32 | ns |
| Serine | 66.78 | 77.21 | 73.91 | 3.39 | ns |
| Glutamic acid | 72.06 | 89.31 | 93.58 | 5.19 | ns |
| Glycine | 47.46 | 58.73 | 47.99 | 2.46 | ns |
| Histidine | 57.65 | 69.77 | 74.37 | 3.19 | ns |
| Taurine | 114.34 | 117.04 | 128.12 | 6.58 | ns |
| Arginine | 85.45 | 95.51 | 100.69 | 4.27 | ns |
| Threonine | 99.25 | 117.94 | 114.23 | 4.76 | ns |
| Alanine | 151.09 b | 206.50 a | 166.96 b | 7.66 | ** |
| Proline | 59.83 a | 70.06 a | 44.45 b | 3.17 | ** |
| Cysteine | 34.99 b | 44.05 b | 60.23 a | 3.49 | ** |
| Tyrosine | 85.71 | 98.26 | 103.75 | 3.75 | ns |
| Valine | 132.20 b | 170.32 a | 157.17 a,b | 6.15 | * |
| Methionine | 79.47 b | 102.12 a | 102.56 a | 3.82 | * |
| Lysine | 81.62 | 103.37 | 104.76 | 5.71 | ns |
| Isoleucine | 117.78 b | 148.45 a | 147.91 a | 5.69 | * |
| Leucine | 249.77 b | 315.94 a | 330.03 a | 12.26 | * |
| Phenylalanine | 152.09 b | 187.83 a | 183.80 a | 6.55 | * |
| Total free amino acids | 1690.36 | 2074.54 | 2034.20 | 77.56 | ns |
| Volatile (AU × 104/g of Cecina) | m/z | LRI | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|---|---|
| Cyclobutanol | 44 | 489 | 4.90 | 3.71 | 4.08 | 0.25 | ns |
| Methanethiol | 48 | 492 | 1.37 a,b | 1.27 b | 1.73 a | 0.076 | * |
| Propan-1-ol | 59 | 563 | 21.23 | 23.57 | 17.64 | 1.01 | ns |
| 2-methylpropan-1-ol | 43 | 643 | 5.90 | 5.19 | 6.69 | 0.31 | ns |
| 3-methylbutan-1-ol | 70 | 817 | 133.38 b | 168.32 a | 120.68 b | 7.22 | * |
| 2-methylbutan-1-ol | 57 | 821 | 36.36 | 33.30 | 29.34 | 1.69 | ns |
| Pentan-1-ol | 70 | 859 | 20.35 | 16.81 | 20.39 | 1.01 | ns |
| Propane-1,2-diol | 45 | 887 | 172.17 | 172.08 | 155.07 | 10.05 | ns |
| Hexane-1,6-diol | 59 | 910 | 5.37 | 7.22 | 5.77 | 0.38 | ns |
| Prop-2-en-1-ol | 57 | 933 | 79.92 b | 88.46 b | 130.14 a | 6.71 | ** |
| Hexan-1-ol | 56 | 975 | 37.20 b | 22.30 c | 46.61 a | 2.27 | *** |
| Furan-3-ylmethanol | 98 | 986 | 326.71 | 304.58 | 312.89 | 10.28 | ns |
| Butane-1,2-diol | 59 | 995 | 22.78 b | 32.44 a | 26.29 a,b | 1.57 | * |
| 3-ethyl-4-methylpentan-1-ol | 69 | 1001 | 14.04 a | 17.28 a | 9.86 b | 0.85 | *** |
| Oct-1-en-3-ol | 57 | 1079 | 151.20 | 126.29 | 119.37 | 7.08 | ns |
| 3-methylsulfanylpropan-1-ol | 106 | 1106 | 10.82 | 11.46 | 7.46 | 0.82 | ns |
| Phenylmethanol | 108 | 1159 | 81.48 b | 94.45 a | 79.46 b | 2.64 | * |
| 4-methylphenol | 107 | 1218 | 41.53 | 42.15 | 37.13 | 1.60 | ns |
| 2-phenylethanol | 91 | 1221 | 139.83 | 150.49 | 109.50 | 9.02 | ns |
| Undecan-1-ol | 97 | 1299 | 3.03 | 3.49 | 3.15 | 0.21 | ns |
| 2-methoxy-4-prop-2-enylphenol | 164 | 1394 | 3.33 | 4.09 | 2.99 | 0.28 | ns |
| Total alcohols | 1312.99 | 1328.95 | 1246.23 | 26.32 | ns | ||
| Pentane | 43 | 500 | 8.98 a,b | 7.65 b | 10.36 a | 0.45 | * |
| 3-methylpentane | 56 | 542 | 1.24 | 1.29 | 1.34 | 0.066 | ns |
| Hexane | 57 | 600 | 134.71 | 117.53 | 115.89 | 4.50 | ns |
| 2-methylhexane | 85 | 620 | 0.76 c | 1.22 b | 1.72 a | 0.083 | *** |
| 3-methylhexane | 71 | 633 | 0.71 | 0.89 | 0.86 | 0.045 | ns |
| Heptane | 71 | 700 | 23.76 b | 33.41 a | 36.33 a | 1.04 | *** |
| 3-ethylpentane | 71 | 759 | 422.57 a | 254.19 b | 232.08 b | 32.68 | * |
| 2,3,3-trimethylpentane | 70 | 767 | 395.27 b | 437.89 a | 454.87 a | 6.37 | *** |
| 2,3-dimethylhexane | 70 | 775 | 43.98 b | 101.96 a | 108.49 a | 6.04 | *** |
| (E)-3,4-dimethylhex-2-ene | 83 | 779 | 35.79 c | 41.96 b | 46.82 a | 0.96 | *** |
| 2-methylheptane | 57 | 782 | 4.43 | 3.51 | 4.15 | 0.22 | ns |
| Octane | 85 | 800 | 40.47 | 39.49 | 39.90 | 2.08 | ns |
| 2,3-dimethylheptane | 84 | 906 | 44.24 | 46.68 | 49.30 | 0.97 | ns |
| 3,4-dimethylheptane | 70 | 909 | 14.54 | 15.37 | 15.41 | 0.50 | ns |
| 2,2-dimethylpropane | 57 | 927 | 247.01 | 240.39 | 204.98 | 11.90 | ns |
| 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene | 93 | 1000 | 21.85 a | 15.83 b | 22.74 a | 0.96 | ** |
| Decane | 57 | 1000 | 758.56 | 769.19 | 658.41 | 47.48 | ns |
| Tridecane | 71 | 1300 | 153.55 b | 177.51 a,b | 193.22 a | 6.41 | * |
| Tetradecane | 57 | 1400 | 17.09 a | 14.44 a | 6.48 b | 0.89 | *** |
| Pentadecane | 71 | 1500 | 2.63 | 2.36 | 2.49 | 0.13 | ns |
| Lineal hydrocarbons | 1139.79 | 1161.59 | 1063.09 | 49.89 | ns | ||
| Branched hydrocarbons | 1232.39 | 1161.18 | 1142.76 | 29.37 | ns | ||
| Total hydrocarbons | 2372.14 | 2322.77 | 2205.84 | 66.73 | ns |
| Volatile (AU × 104/g of Cecina) | m/z | LRI | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|---|---|
| Propan-2-one | 58 | 518 | 22.47 b | 40.45 a | 17.44 b | 1.87 | *** |
| Butan-2-one | 72 | 586 | 20.99 | 25.25 | 20.90 | 1.28 | ns |
| Pentan-2-one | 86 | 722 | 2.39 | 2.79 | 2.75 | 0.17 | ns |
| 1-hydroxypropan-2-one | 43 | 726 | 402.56 | 317.67 | 426.42 | 23.03 | ns |
| Pentane-2,3-dione | 100 | 738 | 3.93 | 5.25 | 4.07 | 0.31 | ns |
| 3-hydroxybutan-2-one | 45 | 795 | 1001.61 a,b | 1217.43 a | 835.24 b | 54.50 | * |
| 4-methylpentan-2-one | 100 | 803 | 1.65 | 1.66 | 1.43 | 0.0934 | ns |
| 1-hydroxybutan-2-one | 57 | 873 | 156.20 | 129.66 | 133.20 | 8.99 | ns |
| Cyclopentanone | 84 | 883 | 21.93 | 23.14 | 24.47 | 0.84 | ns |
| Cyclohexanone | 98 | 940 | 13.28 | 13.84 | 13.03 | 0.67 | ns |
| Cyclopent-2-en-1-one | 82 | 947 | 18.86 | 20.60 | 18.67 | 0.94 | ns |
| 3-methylcyclopentan-1-one | 98 | 952 | 5.68 | 6.48 | 4.71 | 0.36 | ns |
| 2-methylcyclopent-2-en-1-one | 96 | 1017 | 107.45 | 116.27 | 105.72 | 5.52 | ns |
| 1-(furan-2-yl)ethanone | 110 | 1026 | 85.81 | 86.07 | 72.84 | 4.21 | ns |
| Oxolan-2-one | 86 | 1072 | 51.96 | 42.73 | 45.35 | 2.05 | ns |
| 3-methylcyclopent-2-en-1-one | 96 | 1095 | 109.65 | 109.99 | 95.31 | 4.59 | ns |
| 1-(furan-2-yl)propan-2-one | 95 | 1117 | 17.07 | 16.15 | 15.27 | 0.83 | ns |
| 4-methyl-2H-furan-5-one | 98 | 1126 | 75.07 | 67.83 | 67.24 | 2.83 | ns |
| 2-hydroxy-3-methylcyclopent-2-en-1-one | 112 | 1144 | 124.93 | 111.59 | 106.67 | 6.54 | ns |
| 2-hydroxy-3,4-dimethylcyclopent-2-en-1-one | 126 | 1164 | 10.05 | 9.40 | 9.18 | 0.44 | ns |
| 3,4,5-trimethylcyclopent-2-en-1-one | 124 | 1167 | 11.88 | 12.24 | 11.06 | 0.64 | ns |
| Phenacyl formate | 105 | 1172 | 11.37 | 11.81 | 10.83 | 0.56 | ns |
| 1-cyclopropylpropan-1-one | 69 | 1206 | 43.35 | 44.37 | 39.63 | 2.33 | ns |
| 1-(3,5-dihydroxyphenyl) ethanone | 137 | 1331 | 6.34 | 7.75 | 5.87 | 0.42 | ns |
| 2,3-dihydroinden-1-one | 104 | 1354 | 2.31 | 2.34 | 2.19 | 0.10 | ns |
| Total ketones | 2328.79 | 2442.78 | 2089.49 | 66.54 | ns | ||
| Methylsulfanylmethane | 62 | 520 | 4.91 b | 6.39 a | 3.45 c | 0.27 | *** |
| Methanedithione | 76 | 524 | 19.73 b | 21.23 a,b | 24.19 a | 0.76 | * |
| Total sulphur compounds | 24.64 | 27.63 | 27.64 | 0.80 | ns | ||
| Methyl acetate | 74 | 529 | 3.03 b | 3.27 b | 4.34 a | 0.19 | ** |
| Ethenyl acetate | 86 | 581 | 109.68 a | 121.71 a | 65.68 b | 6.34 | *** |
| Ethyl propanoate | 57 | 740 | 6.51 b | 10.89 a | 6.76 b | 0.48 | *** |
| 3-methylbutyl acetate | 88 | 930 | 28.76 | 31.26 | 24.53 | 1.77 | ns |
| 3-methylbutyl acetate | 70 | 959 | 13.63 b | 18.12 a,b | 19.28 a | 0.99 | * |
| 2-methylbutyl acetate | 70 | 962 | 2.07 | 3.51 | 3.62 | 0.29 | ns |
| Propyl 3-methylbutanoate | 85 | 1032 | 5.03 | 5.99 | 6.60 | 0.31 | ns |
| Total esters | 168.72 a | 194.75 a | 130.82 b | 6.74 | *** |
| Volatile (AU × 104/g of Cecina) | m/z | LRI | Control | Salt Mixture I | Salt Mixture II | SEM | Sig. |
|---|---|---|---|---|---|---|---|
| 2-methylpropanal | 72 | 548 | 12.81 a,b | 15.30 a | 11.68 b | 0.55 | * |
| 3-methylbutanal | 58 | 656 | 98.89 | 118.39 | 100.64 | 5.49 | ns |
| 2-methylbutanal | 58 | 669 | 67.11 | 83.82 | 72.19 | 3.91 | ns |
| Hexanal | 56 | 879 | 93.84 a | 59.43 b | 82.62 a | 4.71 | ** |
| Furan-2-carbaldehyde | 96 | 947 | 25.13 a | 17.97 b | 30.13 a | 1.59 | ** |
| Benzaldehyde | 105 | 1072 | 110.03 | 134.32 | 108.87 | 9.61 | ns |
| 2-phenylacetaldehyde | 91 | 1152 | 231.46 a | 234.81 a | 146.51 b | 11.30 | *** |
| Nonanal | 82 | 1184 | 10.72 | 8.77 | 8.71 | 0.58 | ns |
| 5-ethylfuran-2-carbaldehyde | 124 | 1232 | 5.61 | 5.59 | 5.52 | 0.36 | ns |
| Hexadecanal | 82 | 1581 | 4.18 | 3.42 | 4.13 | 0.29 | ns |
| Total aldehydes | 659.77 | 681.81 | 571.02 | 22.62 | ns | ||
| 3-methylfuran | 82 | 571 | 1.47 | 1.61 | 1.42 | 0.0880 | ns |
| 2-ethylfuran | 81 | 703 | 6.87 | 6.03 | 5.20 | 0.38 | ns |
| 2,3,5-trimethylfuran | 110 | 872 | 1.51 b | 2.71 a | 0.99 b | 0.20 | *** |
| 2-butylfuran | 81 | 963 | 4.65 | 3.49 | 3.60 | 0.28 | ns |
| 2-pentylfuran | 81 | 1065 | 106.93 a | 66.65 b | 49.03 b | 6.14 | *** |
| 2-ethenylfuran | 94 | 1146 | 95.37 | 87.32 | 83.27 | 3.68 | ns |
| 1- (5-methylfuran-2-yl) ethanone | 109 | 1147 | 20.97 | 21.14 | 19.28 | 1.08 | ns |
| 3-phenylfuran | 144 | 1287 | 2.90 b | 2.86 b | 4.15 a | 0.25 | * |
| Total furans | 240.67 a | 191.81 b | 166.94b | 9.42 | ** | ||
| Acetic acid | 60 | 686 | 1261.07 | 1183.54 | 1143.65 | 51.66 | ns |
| Propanoic acid | 74 | 835 | 111.20 | 95.39 | 95.79 | 5.09 | ns |
| 2-methylpropanoic acid | 73 | 903 | 153.48 a | 159.91 a | 79.96 b | 8.88 | *** |
| Butanoic acid | 60 | 936 | 261.55 | 213.27 | 242.13 | 10.51 | ns |
| 3-methylbutanoic acid | 60 | 994 | 1156.22 a | 1320.02 a | 474.30 b | 66.19 | *** |
| 2-methylbutanoic acid | 74 | 1001 | 417.61 a | 425.84 a | 197.65 b | 23.26 | *** |
| Pentanoic acid | 60 | 1029 | 16.30 | 13.89 | 15.50 | 0.70 | ns |
| 2,2-dimethylpropanoyl 2,2-dimethylpropanoate | 85 | 1062 | 7.47 a | 6.23 a,b | 5.39 b | 0.29 | * |
| Hexanoic acid | 60 | 1113 | 67.77 | 51.03 | 58.06 | 3.26 | ns |
| Total acids | 3452.67 a | 3469.13 a | 2312.42 b | 109.30 | *** | ||
| Toluene | 92 | 812 | 43.41 | 54.41 | 56.22 | 2.52 | ns |
| 1,4-xylene | 106 | 944 | 13.25 | 14.63 | 10.80 | 0.72 | ns |
| 2-methoxyphenol | 109 | 1192 | 573.22 | 546.17 | 511.95 | 23.60 | ns |
| 4-methoxy-3-methylphenol | 138 | 1257 | 26.14 | 25.56 | 21.78 | 1.57 | ns |
| 2,4-dimethylphenol | 107 | 1262 | 9.48 | 9.71 | 8.36 | 0.48 | ns |
| 2-methoxy-5-methylphenol | 138 | 1275 | 230.34 | 238.75 | 210.35 | 11.25 | ns |
| 1,3-dimethoxy-5-methylbenzene | 152 | 1318 | 6.48 | 7.63 | 5.99 | 0.40 | ns |
| 4-ethyl-2-methoxyphenol | 137 | 1338 | 76.28 | 75.63 | 59.34 | 4.51 | ns |
| 2,6-dimethoxyphenol | 154 | 1398 | 31.65 | 32.18 | 26.50 | 1.33 | ns |
| 1,2,3-trimethoxy-5-methylbenzene | 182 | 1414 | 2.61 | 3.48 | 2.85 | 0.15 | ns |
| 2-methoxy-4-prop-1-enylphenol | 164 | 1456 | 2.20 b | 3.02 a | 1.56 b | 0.180 | ** |
| Phenol and benzene-derived compounds | 1015.06 | 1011.20 | 915.71 | 40.34 | ns | ||
| (E)-but-2-enedinitrile | 78 | 641 | 2.92 | 2.57 | 3.03 | 0.18 | ns |
| Mthylimino(oxo)methane | 57 | 732 | 79.87 | 77.24 | 69.76 | 3.58 | ns |
| Pyrazine | 80 | 784 | 11.76 b | 15.16 a,b | 19.10 a | 0.98 | ** |
| Pyridine | 79 | 804 | 10.98 | 10.51 | 13.82 | 0.72 | ns |
| 2,3-dihydro-1,4-dioxine | 86 | 853 | 21.91 | 19.86 | 23.32 | 0.83 | ns |
| Aniline | 93 | 894 | 3.13 | 2.99 | 3.32 | 0.15 | ns |
| Imidazolidine-2,4-dione | 100 | 899 | 3.36 | 3.30 | 3.40 | 0.19 | ns |
| 2,6-dimethylpyrazine | 108 | 1001 | 53.37 b | 91.97 a | 62.30 b | 3.79 | *** |
| Diethylcanamide | 98 | 1005 | 9.71 | 8.63 | 7.29 | 0.53 | ns |
| 2,3-dimethylpyrazine | 108 | 1010 | 31.86 b | 52.34 a | 19.27 c | 2.57 | *** |
| 2,3,5-trimethylpyrazine | 122 | 1088 | 213.23 b | 376.29 a | 179.47 b | 16.63 | *** |
| 1H-imidazole-5-carbaldehyde | 96 | 1196 | 4.61 | 4.82 | 4.46 | 0.25 | ns |
| Total others | 446.71 b | 665.69 a | 408.47 b | 21.08 | *** | ||
| Total compounds | 12022.17 a | 12336.51 a | 10074.59 b | 224.01 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ramella, M.; Lorenzo, J.M.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Campagnol, P.C.B.; Franco, D. Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina. Foods 2021, 10, 669. https://doi.org/10.3390/foods10030669
Vargas-Ramella M, Lorenzo JM, Domínguez R, Pateiro M, Munekata PES, Campagnol PCB, Franco D. Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina. Foods. 2021; 10(3):669. https://doi.org/10.3390/foods10030669
Chicago/Turabian StyleVargas-Ramella, Marcio, José M. Lorenzo, Rubén Domínguez, Mirian Pateiro, Paulo E. S. Munekata, Paulo C. B. Campagnol, and Daniel Franco. 2021. "Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina" Foods 10, no. 3: 669. https://doi.org/10.3390/foods10030669
APA StyleVargas-Ramella, M., Lorenzo, J. M., Domínguez, R., Pateiro, M., Munekata, P. E. S., Campagnol, P. C. B., & Franco, D. (2021). Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina. Foods, 10(3), 669. https://doi.org/10.3390/foods10030669