The Effect of Ilex × meserveae S. Y. Hu Extract and Its Fractions on Renal Morphology in Rats Fed with Normal and High-Cholesterol Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Materials
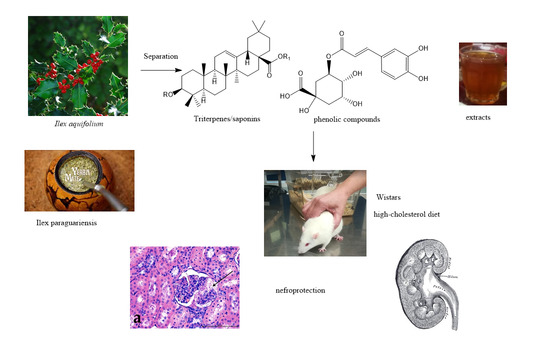
2.2. Plant Extracts
2.2.1. Water Extracts
2.2.2. Polyphenols
2.2.3. Saponins
2.2.4. Terpenoids
2.3. Animals, Housing, and Diets
2.4. Specimen Processing and Staining
2.5. Statistical Analysis
3. Results
3.1. Morphological Studies
3.2. Hematoxylin and Eosin Staining
3.3. Alcian Blue Staining
3.4. Morphometric Analysis
3.4.1. Thickness of the Basement Membrane
3.4.2. Comparison of the Surface of the Glomerular Capsule to the Capillary Tuft
3.4.3. The Ratio between the Size of the Glomerular Capsule and the Capillary Tuft
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Terpenoid Fraction
Appendix A.1.1. Preparation
Appendix A.1.2. Analysis
| KI exp. | KI lit. | RT [min] | Compound | Ilex × meserveae RA [%] |
|---|---|---|---|---|
| 2835 | 2832 | 20.65 | all-trans-Squalene | 0.69 |
| 3162 | 3141 | 24.93 | a-Tocopherol, TMS derivative | 2.21 |
| 3361 | 3370 | 27.78 | (3β)-Olean-18-en-3-ol (TMS) | tr |
| 3370 | 3344 | 27.93 | β-Sitosterol (TMS) | 7.67 |
| 3384 | 3353 | 28.16 | β-Amyrin (TMS) | 9.05 |
| 3397 | 3385 | 28.37 | Germanicol (TMS) | 2.69 |
| 3420 | 3406 | 28.79 | α-Amyrin (TMS) | 28.97 |
| 3427 | 3435 | 28.93 | Lupeol (TMS) | 17.34 |
| 3508 | 3523 | 30.50 | Lupeoyl acetate | 0.75 |
| 3530 | 3540 | 30.99 | Uvaol, 2-O-TMS | 2.35 |
| 3563 | 3560 | 31.73 | (3α)-Lup-20(29)-ene-3,28-diol (O,O-bis-TMS) | 12.13 |
| 3580 | 3588 | 32.11 | Betulinic acid (O,O-bis-TMS) | 1.90 |
| 3596 | 3591 | 32.46 | Oleanolic acid (TMS) | 1.00 |
| 3643 | 3657 | 33.60 | Ursolic acid (TMS) | 5.22 |
| 3650 | n.a. | 33.75 | Maslinic acid * (TMS) | 6.54 |
Appendix A.2. Saponin Fraction
Appendix A.2.1. Preparation
Appendix A.2.2. UHPLC-ESI-MS Conditions of Analysis of Saponins
Appendix A.2.3. Results of UHPLC-ESI-MS Saponin Profiling
| No. | Compound Number Reported in [36] | [M–H]− Measured [m/z] | Proposed Formula | Il.1a | Il.1b | Il.6 | |||
|---|---|---|---|---|---|---|---|---|---|
| RT [min] | RA [%] | RT [min] | RA [%] | RT [min] | RA [%] | ||||
| 1. | — | 1235.61 | C59H96O27 | — | — | — | — | 6.78 | 73.40 |
| 2. | 39a | 1073.56 | C53H86O22 | — | — | — | — | 7.40 | 70.53 |
| 3. | 39a | 1073.56 | C53H86O22 | 8.07 | 60.97 | 8.03 | 10.70 | 8.06 | 100.00 |
| 4. | 39a | 1073.56 | C53H86O22 | 8.10 | 20.01 | 8.07 | 5.07 | 8.10 | 11.53 |
| 5. | 40 | 927.50 | C47H76O18 | 8.24 | 18.63 | 8.22 | 8.24 | 8.26 | 99.22 |
| 6. | 41 | 1381.68 | C65H106O31 | 8.48 | 11.11 | 8.47 | 2.78 | — | — |
| 7. | 43 | 1131.56 | C55H88O24 | 8.70 | 10.70 | — | — | — | — |
| 8. | 45 | 1101.55 | C54H86O23 | 8.85 | 14.20 | — | — | — | — |
| 9. | 47 | 1219.61 | C59H96O26 | 9.10 | 59.09 | 9.11 | 9.11 | 13.99 | |
| 9a. | 48 | 911.50 | C47H76O17 | 9.17 | 5.10 | — | — | 9.21 | 31.68 |
| 10. | 49 | 927.50 | C47H76O18 | 9.45 | 18.28 | 9.42 | 8.86 | 9.45 | 10.62 |
| 11. | 56b | 1235.58 | C62H92O25 | 10.13 | 4.05 | — | — | — | — |
| 12. | 56b | 1235.58 | C62H92O25 | 10.40 | 9.40 | — | — | — | — |
| 13. | 55 | 1057.56 | C53H86O21 | 10.78 | 27.96 | 10.72 | 11.11 | 10.82 | 48.36 |
| 14. | 56b | 1235.58 | C62H92O25 | 11.08 | 4.35 | — | — | — | — |
| 15. | 57 | 1115.57 | C55H88O23 | 11.23 | 20.73 | 11.17 | 7.61 | — | — |
| 16. | 62 | 1057.56 | C53H86O21 | 11.85 | 100.00 | 11.78 | 94.64 | — | — |
| 17. | 64 | 1057.56 | C53H86O21 | 12.09 | 68.77 | 12.04 | 54.22 | — | — |
| 18. | 66c | 911.50 | C47H76O17 | 12.49 | 93.17 | 12.44 | 100.00 | — | — |
| 19. | 66c | 911.50 | C47H76O17 | 12.68 | 11.98 | 12.63 | 7.92 | — | — |
| 20. | 66c | 911.50 | C47H76O17 | 12.91 | 20.45 | 12.85 | 13.00 | — | — |
| 21. | 72 | 1085.56 | C54H86O22 | 13.63 | 8.95 | 13.56 | 2.80 | — | — |
| 22. | 77 | 895.51 | C47H76O16 | 14.34 | 20.19 | 14.28 | 18.10 | — | — |
| 23. | 80 | 895.51 | C47H76O16 | 14.63 | 6.43 | 14.55 | 5.83 | — | — |
| 24. | 83 | 953.52 | C49H78O18 | 14.86 | 30.53 | 14.77 | 25.58 | — | — |
| 25. | 82d | 1219.59 | C59H96O26 | 16.24 | 3.25 | 16.21 | 3.55 | — | — |
| 26. | 82d | 1219.59 | C59H96O26 | 16.54 | 2.84 | 16.53 | 2.26 | — | — |
| 27. | 108e | 895.51 | C47H76O16 | 20.36 | 3.68 | 20.36 | 4.03 | — | — |
| 28. | 108e | 895.51 | C47H76O16 | 20.50 | 2.34 | 20.50 | 1.83 | — | — |
| 29. | 110 | 749.45 | C41H66O12 | 22.18 | 3.33 | 22.15 | 3.42 | — | — |
| Compound | Source | MS/MS Interpretation | Probable Identification |
|---|---|---|---|
| 1 Rt = 6.8 min; calc. [M–H]– = 1235.6061, err. 0.5 ppm; neutral formula: C59H96O27 | Il.5 Il.6 | 911 [M–(Hex+Hex)–H]– 765 [M–(Hex+Hex)–dxHex–H]– 749 [M–(Hex+Hex)–Hex–H]– 731 [M–(Hex+Hex)–Hex–18–H]– 603 [M–(Hex+Hex)–Hex–dxHex–H]– 471 [M–(Hex+Hex)–Hex–(dxHex+Pen)–H]– | kudinoside N (SA) |
| 2 Rt = 7.4 min; calc. [M–H]– = 1073.5538, err. −0.1 ppm; neutral formula: C53H86O22 | Il.6 | 749 [M–(Hex+Hex)–H]– 731 [M–(Hex+Hex)–18–H]– 453 [M–(Hex+Hex+18)–(dxHex+Pen)–H]– | latifoloside L (PA) matesaponin 3 (UA) |
| 3/4 Rt = 8.0 min/8.1 min calc. [M–H]– = 1073.5538, err. −0.3 ppm; neutral formula: C53H86O22 | Il.1 Il.5 Il.6 | 911 [M–Hex–H]– 765 [M–Hex–dxHex–H]– 749 [M–Hex–Hex–H]– 731 [M–Hex–Hex–18–H]– 603 [M–Hex–(Hex+dxHex)–H]– 471 [M–Hex–(Hex+dxHex)–Pen–H]– | latifoloside L (PA) matesaponin 3 (UA) |
| 5 Rt = 8.3 min; calc. [M–H]– = 927.4959, err. 0.2 ppm; neutral formula: C47H76O18 | Il.1 Il.5 Il.6 | 765 [M–Hex–H]– 603 [M–Hex–Hex–H]– 471 [M–Hex–Hex–Pen–H]– | ilexoside XV (SA) ilexoside II (PA) ilekudinoside E (PA) ilexsaponin B3 (IG-B) |
| 9 Rt = 9.1 min; calc. [M–H]– = 1219.6117, err. –0.2 ppm; neutral formula: C59H96O26 | Il.1 Il.5 Il.6 | 895 [M–(Hex+Hex)–H]– 749 [M–(Hex+Hex)–dxHex–H]– 733 [M–(Hex+Hex)–Hex–H]– 715 [M–(Hex+Hex)–Hex–18–H]– 587 [M–(Hex+Hex)–(Hex+dxHex)–H]– 569 [M–(Hex+Hex)–(Hex+dxHex)–18–H]– 455 [M–(Hex+Hex)–(Hex+dxHex)–Pen–H]– | matesaponin 4 (UA) |
| 13 RT = 10.8 min; calc. [M–H]– = 1057.5589, err. 0.1 ppm; neutral formula: C53H86O21 | Il.1 Il.5 Il.6 | 733 [M–(Hex+Hex)–H]– 587 [M–(Hex+Hex)–dxHex–H]– 455 [M–(Hex+Hex)–dxHex–Pen–H]– | matesaponin 2 (UA) or isomer |
| 16 RT = 11.85 min; calc. [M–H]– = 1057.5589, err. 0.5 ppm; neutral formula: C53H86O21 | Il.1 | 895 [M–Hex–H]– 749 [M–Hex–dxHex–H]– 733 [M–Hex–Hex–H]– 715 [M–Hex–Hex–18–H]– 587 [M–Hex–(Hex+dxHex)–H]– 569 [M–Hex–(Hex+dxHex)–18–H]– 455 [M–Hex–(Hex+dxHex)–Pen–H]– | matesaponin 2 (UA) ilekudinoside A (OA) |
| 17 RT = 12.09 min; calc. [M–H]– = 1057.5589, err. –0.5 ppm; neutral formula: C53H86O21 | Il.1 | 895 [M–Hex–H]– 749 [M–Hex–dxHex–H]– 733 [M–Hex–Hex–H]– 715 [M–Hex–Hex–18–H]– 587 [M–Hex–(Hex+dxHex)–H]– 569 [M–Hex–(Hex+dxHex)–18–H]– 455 [M–Hex–Hex–dxHex–Pen–H]– | ilekudinoside A (OA) |
| 18 RT = 12.49 min; calc. [M–H]– = 911.5010, err. –1.6 ppm; neutral formula: C47H76O17 | Il.1 | 749 [M–Hex–H]– 587 [M–Hex–Hex–H]– 569 [M–Hex–Hex–18–H]– 455 [M–Hex–Hex–Pen–H]– | matesaponin 1 (UA) guaiacin B (OA) |
| 24 RT = 14.86min; calc. [M–H]– = 953.5115, err. –1.1 ppm; neutral formula: C49H78O18 | Il.1 | 749 [M–Hex(Ac)–H]– 731 [M–Hex(Ac)–18–H]– 629 [M–Hex–Hex–H]– 587 [M–Hex(Ac)–Hex–H]– 569 [M–Hex(Ac)–Hex–18–H]– 455 [M–Hex–Hex–Pen–H]– | I. amara saponin (UA), 77-52-1 I. amara saponin (OA), 508-02-1 |
| Group No | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| I | - | * | * | * | * | 0 |
| II | * | - | * | * | * | * |
| III | * | * | - | 0 | 0 | * |
| IV | * | * | 0 | - | 0 | * |
| V | * | * | 0 | 0 | - | * |
| VI | 0 | * | * | * | * | - |
| Group No | Ia | IIa | IIIa | IV | Va | VIa |
|---|---|---|---|---|---|---|
| Ia | - | 0 | 0 | * | 0 | 0 |
| IIa | 0 | - | 0 | * | 0 | 0 |
| IIIa | 0 | 0 | - | * | 0 | 0 |
| IVa | * | * | * | - | * | * |
| V | 0 | 0 | 0 | * | - | 0 |
| VIa | 0 | 0 | 0 | * | 0 | - |
| Group No | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| I | - | * | * | * | * | 0 |
| II | * | - | * | * | * | * |
| III | * | * | - | * | 0 | * |
| IV | * | * | * | - | * | * |
| V | * | * | 0 | * | - | * |
| VI | 0 | * | * | * | * | - |
| Group No | Ia | IIa | IIIa | IV | Va | VIa |
|---|---|---|---|---|---|---|
| Ia | - | * | * | * | * | * |
| IIa | * | - | 0 | * | * | * |
| IIIa | * | 0 | - | * | * | * |
| IVa | * | * | * | - | 0 | * |
| V | * | * | * | 0 | - | * |
| VIa | * | * | * | * | * | - |
| Group No | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| I | - | * | * | 0 | * | 0 |
| II | * | - | * | * | * | * |
| III | * | * | - | * | * | * |
| IV | 0 | * | * | - | * | 0 |
| V | * | * | * | * | - | 0 |
| VI | 0 | * | * | 0 | 0 | - |
| Group No | Ia | IIa | IIIa | IV | Va | VIa |
|---|---|---|---|---|---|---|
| Ia | - | * | 0 | 0 | * | 0 |
| IIa | * | - | * | * | 0 | * |
| IIIa | 0 | * | - | 0 | * | 0 |
| IVa | 0 | * | 0 | - | * | 0 |
| V | * | 0 | * | * | - | * |
| VIa | 0 | * | 0 | 0 | * | - |
References
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and functional food as future food: A review. Der Pharm. Lett. 2010, 2, 106–116. [Google Scholar]
- Cogoi, L.; Giacomino, M.S.; Pellegrino, N.; Anesini, C.; Filip, R. Nutritional and phytochemical study of Ilex paraguariensis fruits. J. Chem. 2013, 2013, 750623. [Google Scholar] [CrossRef]
- Bracesco, N.; Sanchez, A.; Contreras, V.; Menini, T.; Gugliucci, A. Recent advances on Ilex paraguariensis research: Minireview. J. Ethnopharmacol. 2011, 136, 378–384. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W. Effects of diet on high-density lipoprotein cholesterol. Curr. Atheroscler. Rep. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Long, Y.; Jiang, X.; Liu, Z.; Wang, D.; Zhao, Y.; Li, D.; Sun, B.-l. Beneficial effects of yerba mate tea (Ilex paraguariensis) on hyperlipidemia in high-fat-fed hamsters. Exp. Gerontol. 2013, 48, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Arçari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; Funck, A.; Pedrazzoli, J.; de Souza, M.F.; Saad, M.J.; Bastos, D.H.; Gambero, A. Antiobesity effects of yerba mate extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity 2009, 17, 2127–2133. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Hao, L.; Fu, X.; Huang, M.; Li, R. Severe hypertriglyceridemia and hypercholesterolemia accelerating renal injury: A novel model of type 1 diabetic hamsters induced by short-term high-fat/high-cholesterol diet and low-dose streptozotocin. BMC Nephrol. 2015, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Gluba-Brzozka, A.; Franczyk, B.; Rysz, J. Cholesterol Disturbances and the Role of Proper Nutrition in CKD Patients. Nutrients 2019, 18, 2820. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L.; Goya, L.; Lecumberri, E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007, 40, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Balzan, S.; Hernandes, A.; Reichert, C.L.; Donaduzzi, C.; Pires, V.A.; Junior, A.G.; Junior, E.L.C. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 2013, 86, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Anesini, C.; Turner, S.; Cogoi, L.; Filip, R. Study of the participation of caffeine and polyphenols on the overall antioxidant activity of mate (Ilex paraguariensis). LWT-Food Sci. Technol. 2012, 45, 299–304. [Google Scholar] [CrossRef]
- Zwyrzykowska, A.; Kupczyński, R.; Jarosz, B.; Szumny, A.; Kucharska, A.Z. Qualitative and quantitative analysis of polyphenolic compounds in Ilex sp. Open Chem. 2015, 13, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Salmon, A.H.; Neal, C.R.; Harper, S.J. New aspects of glomerular filtration barrier structure and function: Five layers (at least) not three. Curr. Opin. Nephrol. Hypertens. 2009, 18, 197–205. [Google Scholar]
- Jarad, G.; Miner, J.H. Update on the glomerular filtration barrier. Curr. Opin. Nephrol. Hypertens. 2009, 18, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.H. The glomerular basement membrane. Exp. Cell Res. 2012, 318, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Jarad, G.; Cunningham, J.; Shaw, A.S.; Miner, J.H. Proteinuria precedes podocyte abnormalities inLamb2–/–mice, implicating the glomerular basement membrane as an albumin barrier. J. Clin. Investig. 2006, 116, 2272–2279. [Google Scholar] [CrossRef] [Green Version]
- de Morais, E.C.; Stefanuto, A.; Klein, G.A.; Boaventura, B.C.; de Andrade, F.; Wazlawik, E.; di Pietro, P.F.; Maraschin, M.; da Silva, E.L. Consumption of yerba mate (Ilex paraguariensis) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. J. Agric. Food Chem. 2009, 57, 8316–8324. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Szumny, A.; Gleńsk, M. Lanostane-type saponins from Vitaliana primuliflora. Molecules 2019, 24, 1606. [Google Scholar] [CrossRef] [Green Version]
- de Resende, P.E.; Verza, S.G.; Kaiser, S.; Gomes, L.F.; Kucharski, L.C.; Ortega, G.G. The activity of mate saponins (Ilex paraguariensis) in intra-abdominal and epididymal fat, and glucose oxidation in male Wistar rats. J. Ethnopharmacol. 2012, 144, 735–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, E.; Lima, N.; Conceição, E.; Peixoto-Silva, N.; Moura, E.; Lisboa, P. Treatment with Ilex paraguariensis (yerba mate) aqueous solution prevents hepatic redox imbalance, elevated triglycerides, and microsteatosis in overweight adult rats that were precociously weaned. Braz. J. Med. Biol. Res. 2018, 51, e7342. [Google Scholar] [CrossRef] [PubMed]
- Gebara, K.S.; Gasparotto Junior, A.; Palozi, R.A.; Morand, C.; Bonetti, C.I.; Gozzi, P.T.; de Mello, M.R.; Costa, T.A.; Cardozo Junior, E.L. A randomized crossover intervention study on the effect a standardized mate extract (Ilex paraguariensis A. St.-Hil.) in men predisposed to cardiovascular risk. Nutrients 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, T.R.; Roman, S.S.; Dal Pra, V.; Cansian, R.L.; Mossi, A.J.; Oliveira, V.J.; Mazutti, M.A. Acute toxicity and anti-inflammatory effects of supercritical extracts of Ilex paraguariensis. Afr. J. Pharm. Pharmacol. 2011, 5, 1162–1169. [Google Scholar]
- Görgen, M.; Turatti, K.; Medeiros, A.R.; Buffon, A.; Bonan, C.D.; Sarkis, J.J.; Pereira, G.S. Aqueous extract of Ilex paraguariensis decreases nucleotide hydrolysis in rat blood serum. J. Ethnopharmacol. 2005, 97, 73–77. [Google Scholar] [CrossRef]
- De Andrade, F.; de Albuquerque, C.A.C.; Maraschin, M.; da Silva, E.L. Safety assessment of yerba mate (Ilex paraguariensis) dried extract: Results of acute and 90 days subchronic toxicity studies in rats and rabbits. Food Chem. Toxicol. 2012, 50, 328–334. [Google Scholar] [CrossRef]
- Kataoka, S.; Norikura, T.; Sato, S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. J. Nutr. Biochem. 2018, 56, 99–108. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Yehia, H.M.; AL-Megrin, W.A.; Akabawy, A.M.; Amin, H.K.; Moneim, A.E.A. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods 2020, 75, 104202. [Google Scholar] [CrossRef]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic acid promotes development of brown adipocyte-like phenotype in 3T3-l1 adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; He, X.-W.; Jiang, J.-G.; Xu, X.-L. Efficacy evaluation of a Chinese bitter tea (Ilex latifolia Thunb.) via analyses of its main components. Food Funct. 2014, 5, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Arçari, D.P.; Bartchewsky Jr, W.; dos Santos, T.W.; Oliveira, K.A.; de Oliveira, C.C.; Gotardo, É.M.; Pedrazzoli Jr, J.; Gambero, A.; Ferraz, L.F.; Carvalho, P.d.O. Anti-inflammatory effects of yerba mate extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol. Cell. Endocrinol. 2011, 335, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Maria-Ferreira, D.; Dartora, N.; da Silva, L.M.; Pereira, I.T.; de Souza, L.M.; Ritter, D.S.; Iacomini, M.; Werner, M.F.D.; Sassaki, G.L.; Baggio, C.H. Chemical and biological characterization of polysaccharides isolated from Ilex paraguariensis A. St.-Hil. Int. J. Biol. Macromol. 2013, 59, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dartora, N.; de Souza, L.M.; Paiva, S.M.M.; Scoparo, C.T.; Iacomini, M.; Gorin, P.A.J.; Rattmann, Y.D.; Sassaki, G.L. Rhamnogalacturonan from Ilex paraguariensis: A potential adjuvant in sepsis treatment. Carbohydr. Polym. 2013, 92, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.P.; Quaggin, S.E. The cell biology of renal filtration. J. Cell Biol. 2015, 209, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Rops, A.L.W.M.M.; van der Vlag, J.; Lensen, J.F.M.; Wijnhoven, T.J.M.; van den Heuvel, L.P.W.J.; van Kuppevelt, T.H.; Berden, J.H.M. Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int. 2004, 65, 768–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrin, A.; Long, C.; Motley, T.J.; Kennelly, E.J. LC-MS metabolomics and chemotaxonomy of caffeine-containing Holly (Ilex) species and related taxa in the Aquifoliaceae. J. Agric. Food Chem. 2019, 67, 5687–5699. [Google Scholar] [CrossRef]









| Group | Diet Type (8 Animals in Each Diet Group) |
|---|---|
| I | rats fed with a standard diet |
| Ia | rats fed as a group I but with the addition of 20 g of cholesterol per kilogram of diet |
| II | rats receiving instead of drinking water the water extract of I. paraguariensis (each day, freshly infused extract was prepared by extraction of 50 g of leaves with 1L boiled water; every two animals had free access to 250 mL of this sole source of drink per day) |
| IIa | rats fed as group II but with the addition of 20 g of cholesterol per kilogram of diet |
| III | rats receiving instead of drinking water the water extract of I. meserveae “Blue Angel” (each day, freshly infused extract was prepared by extraction of 50 g of leaves with 1L boiled water; every two animals had free access to 250 mL of this sole source of drink per day) |
| IIIa | rats fed as group III but with the addition of 20 g of cholesterol per kilogram of diet |
| IV | rats receiving additionally polyphenol fraction from I. meserveae “Blue Angel” (each day, the dry extract was freshly solubilized in water in a dose of 10 mg/kg BW; every two animals had free access to 250 mL of this sole source of drink per day) |
| IVa | rats fed as group IV but with the addition of 20 g of cholesterol per kilogram of diet |
| V | rats receiving additionally terpenoid fraction from I. meserveae “Blue Angel” (each day, 200 mL of oil was mixed with terpenoids (in a dose 10 mg/kg BW) and 1 kg of feed and left overnight; every two animals had free access to diet and drinking water, supplied ad libitum as in group I) |
| Va | rats fed as group V but with the addition of 20 g of cholesterol per kilogram of diet |
| VI | rats receiving additionally saponin fraction from I. meserveae “Blue Angel” (each day, the dry extract was freshly solubilized in water in a dose of 10 mg/kg BW; every two animals had free access to 250 mL of this sole source of drink per day) |
| VIa | rats fed as group VI but with the addition of 20 g of cholesterol per kilogram of diet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuropka, P.; Zwyrzykowska-Wodzińska, A.; Kupczyński, R.; Włodarczyk, M.; Szumny, A.; Nowaczyk, R.M. The Effect of Ilex × meserveae S. Y. Hu Extract and Its Fractions on Renal Morphology in Rats Fed with Normal and High-Cholesterol Diet. Foods 2021, 10, 818. https://doi.org/10.3390/foods10040818
Kuropka P, Zwyrzykowska-Wodzińska A, Kupczyński R, Włodarczyk M, Szumny A, Nowaczyk RM. The Effect of Ilex × meserveae S. Y. Hu Extract and Its Fractions on Renal Morphology in Rats Fed with Normal and High-Cholesterol Diet. Foods. 2021; 10(4):818. https://doi.org/10.3390/foods10040818
Chicago/Turabian StyleKuropka, Piotr, Anna Zwyrzykowska-Wodzińska, Robert Kupczyński, Maciej Włodarczyk, Antoni Szumny, and Renata M. Nowaczyk. 2021. "The Effect of Ilex × meserveae S. Y. Hu Extract and Its Fractions on Renal Morphology in Rats Fed with Normal and High-Cholesterol Diet" Foods 10, no. 4: 818. https://doi.org/10.3390/foods10040818
APA StyleKuropka, P., Zwyrzykowska-Wodzińska, A., Kupczyński, R., Włodarczyk, M., Szumny, A., & Nowaczyk, R. M. (2021). The Effect of Ilex × meserveae S. Y. Hu Extract and Its Fractions on Renal Morphology in Rats Fed with Normal and High-Cholesterol Diet. Foods, 10(4), 818. https://doi.org/10.3390/foods10040818