Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization
Abstract
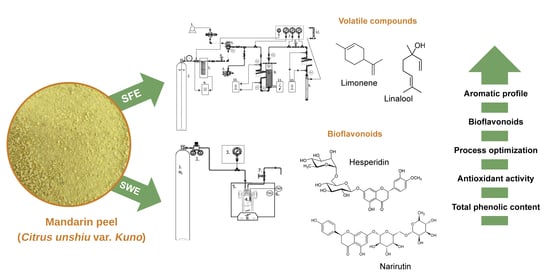
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Material
2.2. Supercritical CO2 (SC-CO2) Extraction
2.3. GC-MS Analysis
2.4. Subcritical Water Extraction (SWE) Technique
2.5. HPLC Analysis
2.6. Determination of Antiradical Activity
2.7. Determination of Total Phenolic Content
2.8. Experimental Design and Process Optimization
3. Results and Discussion
3.1. GC-MS Analysis of SC-CO2 Extracts
3.2. Identification and Quantification of the Compounds in SWE Extracts Using HPLC
3.3. Response Surface Analysis and Process Optimization
3.4. Antiradical Activity and Total Phenolic Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khedkar, R.; Singh, K. Food Industry Waste: A Panacea or Pollution Hazard? In Paradigms in Pollution Prevention, 1st ed.; Jindal, T., Ed.; Springer: Cham, Switzerland, 2018; pp. 35–47. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Ruano, P.; Delgado, L.L.; Picco, S.; Villegas, L.; Tonelli, F.; Merlo, M.E.A.; Rigau, J.; Diaz, D.; Masuelli, M. Extraction and Characterization of Pectins From Peels of Criolla Oranges (Citrus sinensis): Experimental Reviews. In Pectins—Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Cikoš, A.; Jakovljević, M.; Šafranko, S.; Jerković, I. Separation of selected bioactive compounds from orange peel using the sequence of supercritical CO2 extraction and ultrasound solvent extraction: Optimization of limonene and hesperidin content. Sep. Sci. Technol. 2019, 55, 2799–2811. [Google Scholar] [CrossRef]
- Sawamura, M. Citrus Essential Oils: Flavor and Fragrance, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, L.; Tyrrell, H.; Thomas, R.; Matharu, A.; Clark, J.; Hurst, G. Valorization of Waste Orange Peel to Produce Shear-Thinning Gels. J. Chem. Educ. 2019, 96, 3025–3029. [Google Scholar] [CrossRef]
- Teixeira, F.; Novello, D. Physico-chemical. nutritional and sensory aspects of the addition of Citrus fruit by-products in gelation products: A systematic review. Res. Soc. Dev. 2020, 9, e180932669. [Google Scholar] [CrossRef] [Green Version]
- Vidović, S.; Vladić, J.; Nastić, N.; Jokić, S. Subcritical and Supercritical Extraction in Food By-product and Food Waste Valorization. In Innovative Food Processing Technologies, 1st ed.; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 705–721. [Google Scholar]
- Imran, M.; Basharat, S.; Khalid, S.; Aslam, M.; Syed, F.; Jabeen, S.; Kamran, H.; Shahidm, M.Z.; Tufail, T.; Shah, F.-H.; et al. Citrus Peel Polyphenols: Recent Updates and Perspectives. Int. J. Biosci. 2020, 16, 53–70. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.; Ho, W.; Yeap, S.; Sharifudin, S. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2017, 59, 611–625. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hernández Bautista, R.; Sandhu, M.; Hussein, O. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytother. Res. 2014, 29, 323–331. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.; Pons, A.; Sureda, A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2019, 25, 4929–4945. [Google Scholar] [CrossRef]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharm. Res. 2006, 29, 699. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.; et al. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Lachos-Perez, D.; Baseggio, A.; Mayanga-Torres, P.; Maróstica, M.; Rostagno, M.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids. 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.-S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Ko, M.; Kwon, H.; Chung, M. Pilot-scale subcritical water extraction of flavonoids from satsuma mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. Technol. 2016, 38, 175–181. [Google Scholar] [CrossRef]
- Jokić, S.; Horvat, G.; Aladić, K. Design of SFE System Using a Holistic Approach—Problems and Challenges. In Supercritical Fluid Extraction: Technology. Applications and Limitations; Lindy, J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 95–122. [Google Scholar]
- Jerković, I.; Družić, J.; Marijanović, Z.; Gugić, M.; Jokić, S.; Roje, M. GC-FID/MS Profiling of Supercritical CO2 Extracts of Peels from Citrus aurantium, C. sinensis cv. Washington navel, C. sinensis cv. Tarocco and C. sinensis cv. Doppio Sanguigno from Dubrovnik Area (Croatia). Nat. Prod. Commun. 2015, 10, 1315–1318. [Google Scholar] [CrossRef] [Green Version]
- Jokić, S.; Aladić, K.; Šubarić, D. Subcritical water extraction laboratory plant design and application. Annu. Croat. Acad. Eng. 2018, 21, 247–258. Available online: https://hrcak.srce.hr/216152 (accessed on 19 March 2021).
- Molnar, M.; Jerković, I.; Suknović, D.; Bilić Rajs, B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules 2017, 22, 348. [Google Scholar] [CrossRef] [Green Version]
- Baş, D.; Boyacı, I. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Jokić, S.; Bijuk, M.; Aladić, K.; Bilić, M.; Molnar, M. Optimisation of supercritical CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Technol. 2015, 51, 403–410. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Lopresto, C.G.; Meluso, A.; Di Sanzo, G.; Chakraborty, S.; Calabro, V. Process-intensified waste valorization and environmentally friendly D-limonene extraction. Euro-Mediterr. J. Environ. Integr. 2019, 4, 31. [Google Scholar] [CrossRef]
- Teixido, E.; Santos, F.J.; Puignou, L.; Galceran, M.T. Analysis of 5-hydroxymethylfurfural in foods by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1135, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cheigh, C.; Chung, E.; Chung, M. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Extraction of flavanones from immature Citrus unshiu pomace: Process optimization and antioxidant evaluation. Sci. Rep. 2020, 10, 19950. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö. 5-Hydroxymethylfurfural (HMF) formation during subcritical water extraction. Food Sci. Biotechnol. 2018, 27, 981–986. [Google Scholar] [CrossRef]
- Wijngaard, H.; Brunton, N. The Optimization of Extraction of Antioxidants from Apple Pomace by Pressurized Liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Banožić, M.; Škerget, M. Separation of active compounds from tobacco waste using subcritical water extraction. J. Supercrit. Fluids 2019, 153, 104593. [Google Scholar] [CrossRef]
- Khan, M.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compost. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]




| Independent Variable | Symbol | Level | ||
|---|---|---|---|---|
| Low (−1) | Center (0) | High (+1) | ||
| Temperature (°C) | X1 | 130 | 175 | 220 |
| Extraction time (min) | X2 | 5 | 10 | 15 |
| Solvent-solid ratio (mL/g) | X3 | 10 | 20 | 30 |
| No. | Compound | 100 Bar (%) | 300 Bar (%) | No. | Compound | 100 Bar (%) | 300 Bar (%) |
|---|---|---|---|---|---|---|---|
| 1. | α-Pinene | 0.03 | 0.09 | 27. | Undecanal | 0.09 | 0.06 |
| 2. | Sabinene | - | 0.04 | 28. | δ-Elemene | 0.31 | 0.4 |
| 3. | Hexanoic acid | 0.05 | 0.03 | 29. | α-Cubebene | 0.11 | 0.08 |
| 4. | β-Myrcene | 0.13 | 0.54 | 30. | Citronellyl acetate | 0.24 | 0.15 |
| 5. | α-Terpinene | - | 0.04 | 31. | Neryl acetate | 0.43 | 0.28 |
| 6. | p-Cymene | 0.04 | - | 32. | α-Copaene | 1.46 | 0.89 |
| 7. | Limonene | 13.16 | 30.65 | 33. | Geranyl acetate | 0.72 | 0.45 |
| 8. | (Z)-β-ocymene | 0.02 | 0.05 | 34. | β-Elemene | 0.99 | 0.86 |
| 9. | γ-Terpinene | 1.75 | 3.69 | 35. | Dodecanal | 0.23 | 0.13 |
| 10. | cis-Sabinene hydrate | 0.11 | 0.16 | 36. | trans-Caryophyllene | 0.9 | 0.54 |
| 11. | α-Terpinolene | 0.17 | 0.32 | 37. | Valencene | 0.51 | 0.3 |
| 12. | Linalool | 2.18 | 1.58 | 38. | α-Muurolene | 0.63 | - |
| 13. | Nonanal | 0.07 | 0.06 | 39. | Eremophilene | 6.7 | 3.99 |
| 14. | trans-p-mentha-2,8-dien-1-ol | - | 0.02 | 40. | γ-Cadinene | 2.21 | 1.38 |
| 15. | trans-Limonene oxide | 0.02 | 0.02 | 41. | Germacrene B | 1.11 | 0.69 |
| 16. | Citronellal | 0.11 | 0.1 | 42. | Dodecanoic acid | 0.34 | 0.32 |
| 17. | Terpinen-4-ol | 0.22 | 0.16 | 43. | t-Muurulol | 0.07 | 0.06 |
| 18. | Octanoic acid | 0.08 | 0.05 | 44. | 3-Oxo-α-ionol | - | - |
| 19. | α-Terpineol | 2.1 | 1.31 | 45. | α-Sinensal | 0.08 | 0.05 |
| 20. | Decanal | 0.53 | 0.37 | 46. | Tetradecanoic acid | 2.42 | 1.56 |
| 21. | β-Citronelol | 0.19 | 0.13 | 47. | Nootkatone | 0.14 | 0.13 |
| 22. | Perilla aldehyde | 0.43 | 0.3 | 48. | Octadecan-1-ol | 0.18 | 0.17 |
| 23. | Nonanoic acid | 0.08 | 0.06 | 49. | Heptadecanoic acid | 0.19 | 0.4 |
| 24. | p-Mentha-1,8-dien-9-ol | 0.23 | 0.15 | 50. | Linoleic acid | 15.44 | 19.04 |
| 25. | Thymol | 0.09 | 0.08 | 51. | Oleic acid | 2.87 | - |
| 26. | Carvacrol | 0.19 | 0.12 |
| Experimental Design (BBD) | Compound (mg/g of Peels) | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Temperature (°C) | Time (min) | Solvent-Solid Ratio (mL/g) | 5-HMF | Hesperidin | Narirutin | Rutin | Chlorogenic Acid |
| 1. | 175 | 10 | 20 | 4.68 | 9.28 | 3.65 | 3.14 | 0.27 |
| 2. | 175 | 15 | 10 | 4.32 | 8.56 | 1.05 | 0.89 | 1.90 |
| 3. | 130 | 10 | 30 | 0.01 | 9.19 | 3.83 | 1.31 | 0.28 |
| 4. | 220 | 5 | 20 | 10.08 | 0.19 | 0.09 | 0.19 | 58.93 |
| 5. | 175 | 15 | 30 | 9.48 | 14.89 | 4.27 | 3.91 | 8.69 |
| 6. | 175 | 10 | 20 | 6.48 | 10.52 | 3.63 | 3.03 | 3.62 |
| 7. | 220 | 10 | 10 | 5.38 | 1.15 | 0.03 | 0.80 | 21.26 |
| 8. | 130 | 15 | 20 | 0.00 | 11.25 | 4.87 | 1.04 | 0.00 |
| 9. | 130 | 10 | 10 | 0.00 | 3.66 | 1.99 | 0.52 | 0.08 |
| 10. | 175 | 5 | 30 | 7.83 | 15.07 | 4.72 | 4.21 | 0.28 |
| 11. | 175 | 5 | 10 | 3.67 | 5.28 | 1.91 | 1.68 | 0.79 |
| 12. | 175 | 10 | 20 | 6.18 | 9.61 | 3.44 | 2.94 | 1.98 |
| 13. | 175 | 10 | 20 | 6.02 | 11.23 | 3.38 | 2.81 | 1.84 |
| 14. | 220 | 15 | 20 | 6.39 | 0.16 | 0.08 | 0.18 | 54.48 |
| 15. | 130 | 5 | 20 | 0.03 | 7.34 | 3.76 | 1.04 | 0.29 |
| 16. | 175 | 10 | 20 | 6.41 | 11.99 | 5.11 | 4.27 | 9.10 |
| 17. | 220 | 10 | 30 | 14.33 | 0.35 | 0.11 | 0.26 | 68.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043. https://doi.org/10.3390/foods10051043
Šafranko S, Ćorković I, Jerković I, Jakovljević M, Aladić K, Šubarić D, Jokić S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods. 2021; 10(5):1043. https://doi.org/10.3390/foods10051043
Chicago/Turabian StyleŠafranko, Silvija, Ina Ćorković, Igor Jerković, Martina Jakovljević, Krunoslav Aladić, Drago Šubarić, and Stela Jokić. 2021. "Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization" Foods 10, no. 5: 1043. https://doi.org/10.3390/foods10051043
APA StyleŠafranko, S., Ćorković, I., Jerković, I., Jakovljević, M., Aladić, K., Šubarić, D., & Jokić, S. (2021). Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods, 10(5), 1043. https://doi.org/10.3390/foods10051043