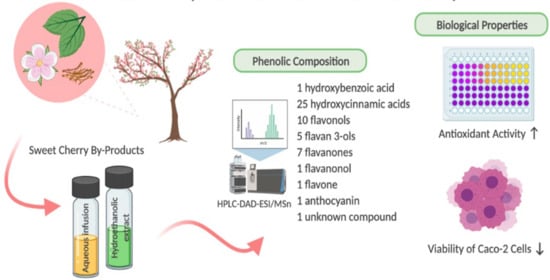
Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Aqueous Infusions and Hydroethanolic Extracts Preparation
2.3. Total Phenolic Compounds Determination
2.4. Total Flanonoids Determination
2.5. Phenolic Compounds Composition
2.5.1. HPLC-DAD-ESI/MSn
2.5.2. HPLC-DAD
2.6. 2.2-Diphenyl-1-Picrylhydrazil Radical (DPPH•)-Scavenging Activity
2.7. Caco-2 Cell Culture and Treatment with By-products Extracts
2.7.1. MTT Assay
2.7.2. LDH Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolics Content
3.2. Total Flavonoids Content
3.3. Analysis of Phenolic Compounds.
3.3.1. Hydroxybenzoic Acids
3.3.2. Hydroxycinnamic Acids
3.3.3. Flavonols
3.3.4. Flavan 3-ols
3.3.5. Flavanones
3.3.6. Flavanonols
3.3.7. Flavone
3.3.8. Anthocyanins
3.4. 2,2-Diphenyl-1-Picrylhydrazil Radical (DPPH•)-Scavenging Activity
3.5. Antiproliferative Effect on Caco-2-Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, L.R. Nutrients, Bioactive Compounds and Bioactivity: The Health Benefits of Sweet Cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2018, 14, 1–20. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Duenas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, C.F.R.F. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food. Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.; Rodrigues, M.; Santos, A.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.C.; Campos, G.; Alves, G.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical compositon of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Silva, G.R.; Vaz, C.V.; Catalão, B.; Ferreira, S.; Cardoso, H.J.; Duarte, A.P.; Socorro, S. Sweet Cherry Extract Targets the Hallmarks of Cancer in Prostate Cells: Diminished Viability, Increased Apoptosis and Suppressed Glycolytic Metabolism. Nutr. Cancer 2020, 72, 917–931. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Almeira, A.P.C.; Bronze, M.R.; Alves, P.M.; De Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agent. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food. Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.M.; Silva, L.R. Assessing the phenolic profile, antioxidant, antidiabetic and protective effects against oxidative damage in human erythrocytes of peaches from Fundão. J. Funct. Foods 2018, 43, 224–233. [Google Scholar] [CrossRef]
- Prvulovic, D.; Popovic, M.; Malenčić, Đ.; Ljubojevic, M.; Ognjanov, V. Phenolic compounds in sweet cherry (Prunus avium L.) petioles and their antioxidant properties. Res. J. Agric. Sci. 2011, 43, 198–202. [Google Scholar]
- Cagno, R.D.; Surico, R.F.; Minervini, G.; Rizzello, C.G.; Lovino, R.; Servili, M.; Taticchi, A.; Urbani, S.; Gobbetti, M. Exploitation of sweet cherry (Prunus avium L.) puree added of stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol. 2011, 28, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Hooman, N.; Mojab, F.; Nickavar, B.; Pouryousefi-Kermani, P. Diuretic effect of powdered Cerasus avium (cherry) tails on healthy volunteers. Pak. J. Pharm. Sci. 2009, 22, 381–383. [Google Scholar]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Bilek, S.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–49. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009, 115, 1364–1372. [Google Scholar] [CrossRef]
- Luís, A.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a source of antioxindar and antimicrobial polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migues, I.; Baenas, N.; Gironés-Vilaplana, A.; Cesio, M.V.; Heinzen, H.; Moreno, D.A. Phenolic Profiling and Antioxidant Capacity of Eugenia uniflora L. (Pitanga) Samples Collected in Different Uruguayan Locations. Foods 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Assessing Jasminum grandiflorum L. authenticity by HPLC-DAD-ESI/MSn and effects on physiological enzymes and oxidative species. J. Pharm. Biomed. Anal. 2014, 88, 157–161. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Souza, V.R.; Pereira, P.A.P.; Silva, T.L.T.; Lima, L.C.O.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–836. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Yüksekkaya, Ş.; Başyiğit, B.; Sağlam, H.; Pekmez, H.; Cansu, Ü.; Karaaslan, A.; Karaaslan, M. Valorization of fruit processing by-products: Free, esterifed, and insoluble bound phytochemical extraction from cherry (Prunus avium) tissues and their biological activities. J. Food Meas Charact. 2021, 15, 1092–1107. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Matteo, A.D.; Russo, R.; Graziani, G.; Ritieni, A.; Vaio, C.D. characterization of autochthonous sweet cherry cultivars (Prunus Avium L.) of Southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Zidova, P.; Paprstein, F. Comparison of Old Cherry Cultivars Grown in Czech Republic by Chemical Composition and Bioactive Compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. analyses of chlorogenic acids and related cinnamic acid derivatives from nicotiana tabacum tissues with the Aid of UPLC-QTOF-MS/MS Based on the In-Source Collision-Induced Dissociation Method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, S.; Maro, A.D.; Petriccione, M.; Galasso, S.; Piccolella, S.; Giuseppe, A.M.A.D.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, A.; Seabra, I.; Braga, M.; Bronze, M.; Sousa, H.; Duarte, C. Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds. J. Supercrit. Fluids. 2010, 55, 184–191. [Google Scholar] [CrossRef]
- Treutter, D.; Feucht, W.; Schmid, P.P.S. Ageing-dependent responses of phloem flavonoids of Prunus avium graftings: Flavanone-, flavone- and isoflavone-glucosides. Sci. Hortic. 1987, 32, 183–193. [Google Scholar] [CrossRef]
- Hasegawa, M. Flavonoids of Various Prunus Species. VI. The Flavonoids in the Wood of Prunus aequinoctialis, P. nipponica, P. Maximowiczii and P. avium. J. Am. Chem. Soc. 1957, 79, 1738–1740. [Google Scholar] [CrossRef]
- Geibel, M.; Geiger, H.; Treutter, D. Tectochrysin 5- and genistein 5-glucosides from the bark of Prunus cerasus. Phytochemistry 1990, 29, 1351–1353. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Antioxidant Polyphenols from Tart Cherries (Prunus cerasus). J. Agric. Food Chem. 1999, 47, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Koksal, E.; Gulçin, I.; Bilsel, G.; Goren, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Kutlu, T.; Takim, K.; Çeken, B.; Kizil, M. DNA damage protecting activity and in vitro antioxidant potential of the methanol extract of Cherry (Prunus avium L). J. Med. Plants Res. 2014, 8, 715–726. [Google Scholar]
- Galati, G.; O’Brien, P. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Yen, G.; Chen, C.; Chang, W.; Wu, M.; Cheng, F.; Shiau, D.; Hsu, C. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic components. J. Food Drug. Anal. 2018, 26, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. In Vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef]

| Phenolic Compound | Calibration Curve Equation | R2 |
|---|---|---|
| Caffeic acid | y = 233.49x + 185.44 | 0.999 |
| Catechin | y = 34.52x + 134.38 | 0.998 |
| Chlorogenic acid | y = 46.37x + 170.34 | 0.999 |
| Chrysin | y = 69.375x − 43.531 | 0.999 |
| p-coumaric acid | y = 260.73x -137.54 | 0.999 |
| Ferulic acid | y = 136.37x + 154.91 | 0.999 |
| Gallic acid | y = 54.13x + 131.11 | 0.997 |
| p-hydroxybenzoic acid | y = 36.18x + 121.90 | 0.995 |
| Kaempferol 3-O-rutinoside | y = 41.442x + 184.11 | 0.999 |
| Naringenin 7-O-glucoside | y = 41.039x + 183.36 | 0.999 |
| Quercetin | y = 48.56x − 11.222 | 0.999 |
| Leaves | Stems | Flowers | |
|---|---|---|---|
| Total Phenols Content (mg GAE per g dw) | 100.71 ± 8.30 | 301.38 ± 5.91 a | 81.20 ± 2.75 a,b |
| Extracts | Total Flavonoids Content (mg QE per g dw) | |
|---|---|---|
| Leaves | Infusion | 31.63 ± 2.24 |
| Hydroethanolic | 35.17 ± 2.62 | |
| Stems | Infusion | 9.93 ± 1.19 a,b |
| Hydroethanolic | 15.25 ± 1.18 a,b,c | |
| Flowers | Infusion | 22.67 ± 0.73 a,b,c,d |
| Hydroethanolic | 24.62 ± 0.39 a,b,c,d |
| Peak | Phenolic Compounds | HPLC-DAD-ESI-MSn Characteristics | |||
|---|---|---|---|---|---|
| Rt (min) | λmax (nm) | Molecular Ion [M + H] (m/z) | Fragments MS/MS (m/z) | ||
| 1 | Feruloyl di-hexose | 13.1 | 320 | 518 | 355/356, 337, 193, 176 |
| 2 | Caffeoylquinic acid derivative 1 | 13.7 | 320 | 371 | 353, 191, 135 |
| 3 | Feruloyl hexose | 14.2 | 320 | 355 | 193, 176 |
| 4 | Caffeoylquinic acid glycoside-derivative | 15.3 | 320 | 515 | 341, 335, 179, 191 |
| 5 | Protocatechuic acid-glycoside | 15.7 | 280 | 315, dimer adduct 631 | 153 |
| 6 | Caffeoylquinic acid derivative 2 | 16.1 | 350 | 503 | 341, 179 |
| 7 | Caffeoylquinic acid-glycoside | 16.4 | 320 | 515 | 341, 179 |
| 8 | Caffeoyl hexose derivative 1 | 17.2 | 350 | 683 | 521, 529, 341, 315, 179 |
| 9 | 3-Caffeoylquinic acid cis | 17.6 | 320 | 353, dimer adduct 707 | 191, 179, 135 |
| 10 | 3,5-diCaffeoylquinic acid 1 | 18.2 | 320 | 515 | 353, 191, 179, 135 |
| 11 | Catechin hexoside | 18.4 | 280 | 451 | 289, 245 |
| 12 | Caffeoyl hexose | 18.7 | 320 | 341 | 179, 135 |
| 13 | Procyanidin dimer B type 1 | 19.2 | 280 | 577 | 289, 407, 425, 451 |
| 14 | p-Coumaroylquinic acid derivative | 19.6 | 320 | 679 | 337, 517, 337, 191, 162 |
| 15 | diCaffeoylquinic acid 1 | 19.8 | 320 | 515 | 323, 191, 353 |
| 16 | p-Coumaric acid derivative | 20.1 | 320 | 337, dimer adduct 675 | 162,191 |
| 17 | 4-Caffeoylquinic acid | 20.8 | 320 | 353, dimer adduct 707 | 173 |
| 18 | Procyanidin tetramer | 21.0 | 280 | 1153 | 865, 577, 289, 245 |
| 19 | Procyanidin trimer | 21.2 | 280 | 865 | 577, 289, 245 |
| 20 | Feruloylquinic acid | 21.2 | 320 | 367 | 193 |
| 21 | 5-Caffeoylquinic acid trans | 21.5 | 320 | 353 | 191, 179 |
| 22 | Procyanidin dimer B type 2 | 22.0 | 280 | 577 | 425, 289, 407, 451 |
| 23 | Kaempferol O-rutinoside-O-hexoside | 22.7 | 350 | 755 | 593, 285, 695 |
| 24 | Cyanidin 3-O-rutinoside | 23.3 | 280 | 595 | 449, 287 |
| 25 | Quercetin 3-O-rutinoside-O-hexoside | 24.4 | 350 | 771 | 609, 463, 301 |
| 26 | Quercetin di-hexoside | 25.0 | 350 | 625 | 453, 301 |
| 27 | Kaempferol O-rutinoside-O-hexoside derivative | 25.3 | 350 | 771 | 593, 285, 327 |
| 28 | p-Coumaroylquinic acid | 25.5 | 320 | 337, dimer adduct 675 | 337, 191 |
| 29 | Unknown 1 | 25.5 | 360 | 465 | 285 |
| 30 | Kaempferol-di-hexoside | 25.6 | 350 | 609 | 285, 447 |
| 31 | Aromandendrine O-hexoside | 26.5 | 350 | 447 | 287 |
| 32 | Quercetin-3-O-hexoside | 28.0 | 350 | 463 | 301, 271, 179 |
| 33 | 3,4-diCaffeoylquinic acid | 28.5 | 320 | 515 | 353, 173 |
| 34 | Kaempferol 3-O-rutinoside | 29.2 | 350 | 593 | 285, 256 |
| 35 | 3,5-diCaffeoylquinic acid 2 | 30.0 | 320 | 515 | 353, 191 |
| 36 | Kaempferol 3-hexoside | 30.7 | 350 | 447 | 285 |
| 37 | Sakuranetin 5-O-hexoside derivative 1 | 31.0 | 280 | 447 | 285 |
| 38 | Caffeoyl hexose derivative 2 | 31.2 | 320 | 503 | 341, 179 |
| 39 | 4,5-diCaffeoylquinic acid | 31.3 | 320 | 515 | 353, 179, 173 |
| 40 | Kaempferol 3-O-acetyl-hexoside | 32.7 | 350 | 489 | 285 |
| 41 | Naringenin 7-O-hexoside | 33.0 | 280 | 433 | 271, 151, 313 |
| 42 | Caffeoyl hexose derivative 3 | 33.8 | 320 | 869 | 451, 341 |
| 43 | diCaffeoylquinic acid 2 | 34.7 | 320 | 515 | 353, 335, 191, 173 |
| 44 | Pinocembrin-O- pentosylhexoside | 35.0 | 280 | 549 | 255, 234 |
| 45 | 3-Coumaroyl-5-caffeoylquinic acid | 35.0 | 320 | 499 | 337,163, 173 |
| 46 | 3-Coumaroyl-4-caffeoylquinic acid | 35.3 | 320 | 499 | 337, 353, 173 |
| 47 | Sakuranetin-O-pentosylhexoside | 35.5 | 280 | 579 | 285, 270 |
| 48 | Naringenin hexoside | 35.9 | 280 | 443 | 271 |
| 49 | Quercetin 3-O-rutinoside | 36.9 | 350 | 609 | 301, 271 |
| 50 | Chrysin 7-O-hexoside | 37.0 | 280 | 415 | 253, 208 |
| 51 | Sakuranetin 5-O-hexoside | 38.3 | 280 | 447 | 285 |
| 52 | Sakuranetin 5-O-hexoside derivative 2 | 39.1 | 280 | 593 | 447, 285 |
| Peak | Phenolic Compounds | Leaves | Stems | Flowers | |||
|---|---|---|---|---|---|---|---|
| Infusion | Hydroethanolic | Infusion | Hydroethanolic | Infusion | Hydroethanolic | ||
| 7 | Caffeoylquinic acid-glycoside | nq | nq | 1936.04 ± 18.9 | 2117.33 ± 25.4 c | nd | nd |
| 9 | 3-Caffeoylquinic acid cis | 18,667.85 ± 162.2 | 20,215.87 ± 917.3 a | 196.23 ± 14.1 a,b | 320.23 ± 46.7 a,b | 23,294.66 ± 653.4 a,b,c,d | 15,996.99 ± 335.4 a,b,c,d,e |
| 10 | 3,5-diCaffeoylquinic acid 1 | nq | nq | nq | 158.85 ± 4.7 | 2948.25 ± 29.5 d | 1309.65 ± 47.9 d,e |
| 15 | diCaffeoylquinic acid 1 | 2210.36 ± 99.3 | 3375.96 ± 98.8 a | nd | nd | nq | nq |
| 16 | p-Coumaric acid derivative | 1482.13 ± 14.9 | 1335.62 ± 51.9 a | nd | nd | nd | nd |
| 17 | 4-Caffeoylquinic acid | 908.93 ± 81.95 | 466.37 ± 19.7 a | nd | nd | nd | nd |
| 21 | 5-Caffeoylquinic acid trans | 24,425.04 ± 897.3 | 27,210.54 ± 1415.7 a | 1095.56 ± 167.7 a,b | 1338.68 ± 40.2 a,b | 3841.41 ± 304.04 a,b,c,d | 640.77 ± 28.6 a,b,c,d,e |
| 22 | Procyanidin dimer B type 2 | nq | nq | 7149.5 ± 510.5 | 8810.67 ± 529.2 c | nd | nd |
| 23 | Kaempferol-O-rutinoside-O-hexoside | nq | nq | nd | nd | 5313.35 ± 91.7 | 2676.57 ± 134.9 e |
| 26 | Quercetin di-hexoside | nd | nd | nd | nd | nq | 348.22 ± 6.3 |
| 28 | p-Coumaroylquinic acid | 473.68 ± 6.3 a | 450.79 ± 9.1 | nd | nd | nd | nd |
| 31 | Aromandrine O-hexoside | nd | nd | nq | 172.96 ± 18.9 | nd | nd |
| 32 | Quercetin 3-O-hexoside | nq | nq | 665.76 ± 1.6 | 1025.78 ± 18.2 c | 702.74 ± 12.8 c,d | 555.16 ± 12.9 c,d |
| 34 | Kaempferol 3-O-rutinoside | 1298.58 ± 44.2 | nq | nq | nq | nd | nd |
| 36 | Kaempferol 3-hexoside | nq | 1542.19 ± 112.13 | nd | nd | nq | nq |
| 41 | Naringenin 7-O-hexoside | nd | nd | 1482.67 ± 15.94 | 1940.77 ± 51.2 c | nd | nd |
| 45 | 3-Coumaroyl-5-caffeoylquinic acid | 696.45 ± 10.9 | 905.1 ± 11.9 a | nd | nd | 340.21 ± 0.95 a,b | 305.5 ± 36.2 a,b |
| 46 | 3-Coumaroyl-4-caffeoylquinic acid | 2481.25 ± 51.8 | 3644.97 ± 64.00 a | nd | nd | 327.81 ± 21.1 a,b | nq |
| 48 | Naringenin hexoside | 326.60 ± 56.1 | 689.26 ± 58.5 c | nd | nd | nd | nd |
| 49 | Quercetin 3-O-rutinoside | 6175.93 ± 148.22 | 3653.48 ± 22.7 a | 404.39 ± 9.7 a,b | 767.00 ± 19.4 a,b,c | 2512.03 ± 6.03 a,b,c,d | 1823.94 ± 38.9 a,b,c,d,e |
| 51 | Sakuranetin 5-O-hexoside | 265.89 ± 9.8 | 214.66 ± 10.6 a | nq | nq | nq | nq |
| Σ | 36,142.42 | 63,704.81 | 12,932.15 | 16,181.65 | 39,280.46 | 23,656.8 | |
| Extracts | DPPH• | |
|---|---|---|
| Leaves | Infusion | 55.12 ± 1.11 |
| Hydroethanolic | 51.52 ± 0.84 | |
| Stems | Infusion | 28.41 ± 0.55 |
| Hydroethanolic | 19.04 ± 0.31 a | |
| Flowers | Infusion | 56.64 ± 0.91 |
| Hydroethanolic | 194.1 ± 2.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; A. Moreno, D.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. https://doi.org/10.3390/foods10061185
Nunes AR, Gonçalves AC, Alves G, Falcão A, Garcia-Viguera C, A. Moreno D, Silva LR. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods. 2021; 10(6):1185. https://doi.org/10.3390/foods10061185
Chicago/Turabian StyleNunes, Ana R., Ana C. Gonçalves, Gilberto Alves, Amílcar Falcão, Cristina Garcia-Viguera, Diego A. Moreno, and Luís R. Silva. 2021. "Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability" Foods 10, no. 6: 1185. https://doi.org/10.3390/foods10061185
APA StyleNunes, A. R., Gonçalves, A. C., Alves, G., Falcão, A., Garcia-Viguera, C., A. Moreno, D., & Silva, L. R. (2021). Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods, 10(6), 1185. https://doi.org/10.3390/foods10061185