Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids
Abstract
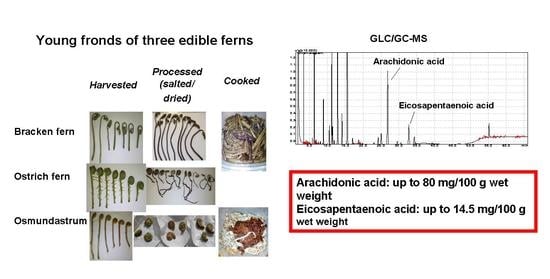
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Lipid Extraction
2.3. Fatty Acid Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Raw Young Fronds
3.2. Processed Fronds
3.3. Cooked Ferns
3.4. Edible Ferns in Comparison with Other Dietary Sources of LC-PUFAs
4. Conclusions
- (1)
- Fiddleheads of three fern species, harvested in the Russian Far East for food consumption, contain the valuable omega-6 and omega-3 long-chain polyunsaturated fatty acids (arachidonic acid, eicosapentaenoic acid, γ-linolenic acid, dihomo-γ-linolenic acids, 20:4n-3) in amounts comparable to certain meats, as well as minor quantities of Δ5-unsaturated polymethylene-interrupted fatty acids (sciadonic and juniperonic acids).
- (2)
- Arachidonic acid always prevailed over eicosapentaenoic acid in terms of its content in the studied species. The highest ratio of omega-6/omega-3 fatty acids, the highest level of ARA and the lowest level of EPA were observed in Pteridium aquilinum. The lowest ratio of omega-6/omega-3 fatty acids, along with the highest percentage of EPA and the lowest one of ARA, among the species was found in Osmundastrum asiaticum, whereas Matteuccia struthiopteris contained a high percentage of ARA, along with a relatively high content of EPA and an intermediate ratio of omega-6/omega-3.
- (3)
- Plant material from fern species collected from two separate Far Eastern regions was different in terms of the percentage of ARA in the total fatty acids, although the ratios of ARA to EPA were similar for each species.
- (4)
- Salted fronds were much more different from freshly harvested fronds in terms of the content of long-chain polyunsaturated fatty acids, compared to that of dried frond samples. Prompt heat treatment (boiling, blanching, drying) of the freshly harvested fronds may be the preferable method for the preservation of long-chain polyunsaturated fatty acids in the processed material.
- (5)
- Valuable long-chain polyunsaturated fatty acids are well preserved in the cooked fern fronds, thus becoming a part of the diet when ferns are included.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, H.A.; Prance, G.T. The ethnobotany of ferns and lycophytes. Fern Gaz. 2015, 20, 1–13. [Google Scholar]
- Khrapko, O.V. Far Eastern ferns: Opportunities for application. Bull. Bot. Gard. Inst. 2007, 1, 81–87. (In Russian) [Google Scholar]
- DeLong, J.M.; Prange, R.K. Fiddlehead fronds: Nutrient rich delicacy. Chron. Hortic. 2008, 48, 12–15. [Google Scholar]
- Liu, Y.; Wujisguleng, W.; Long, C. Food uses of ferns in China: A review. Acta Soc. Bot. Polon. 2012, 81, 263–270. [Google Scholar] [CrossRef]
- Maroyi, A. Not just minor wild edible forest products: Consumption of pteridophytes in sub-Saharan Africa. J. Ethnobiol. Ethnomed. 2014, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Koide, D.; Kadoya, T. Resource amount and cultural legacy affect spatially unbalanced human use of Japan’s non-timber forest products. Ecol. Indic. 2019, 97, 204–210. [Google Scholar] [CrossRef]
- Pemberton, R.W.; Lee, N.S. Wild food plants in South Korea; market presence, new crops, and exports to the United States. Econ. Bot. 1996, 50, 57–70. [Google Scholar] [CrossRef]
- Gerasimenko, N.; Korol, A.; Pikhanova, S.; Gochachko, S. Marketing evaluation of non-wood products of Far Eastern south forests. Pract. Mark. 2003, 3, 17–25. (In Russian) [Google Scholar]
- Tsapalova, I.E.; Pechurina, N.N. Preservation of the salty semifinished fern Matteuccia struthiopteris. Food Proc. Techniq. Technol. 2011, 3, 10. (In Russian) [Google Scholar]
- Nekrasov, E.V.; Svetashev, V.I.; Khrapko, O.V.; Vyssotski, M.V. Variability of fatty acid profiles in ferns: Relation to fern taxonomy and seasonal development. Phytochemistry 2019, 162, 47–55. [Google Scholar] [CrossRef]
- Kawashima, H. Intake of arachidonic acid-containing lipids in adult humans: Dietary surveys and clinical trials. Lipids Health Dis. 2019, 18, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Stessy Bisselou, K.; Kusi Appiah, A.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 fatty acid intake by age, gender, and pregnancy status in the United States: National health and nutrition examination survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Svetashev, V.I.; Imbs, A.B. Isomerization of octadecapentaenoic acid (18:5n-3) in algal lipid samples under derivatization for GC and GC-MS analysis. J. Phycol. 2014, 50, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Svetashev, V.I. Mild method for preparation of 4,4-dimethyloxazoline derivatives of polyunsaturated fatty acids for GC-MS. Lipids 2011, 46, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Bushway, A.A.; Wilson, A.M.; McGann, D.F.; Bushway, R.J. The nutrient composition of fresh fiddlehead greens. J. Food Sci. 1982, 47, 666–667. [Google Scholar] [CrossRef]
- DeLong, J.M.; Hodges, D.M.; Prange, R.K.; Forney, C.F.; Toivenon, P.M.A.; Bishop, M.C.; Elliot, M.L.; Jordan, M.A. The unique fatty acid and antioxidant composition of ostrich fern (Matteuccia struthiopteris) fiddleheads. Can. J. Plant Sci. 2011, 91, 919–930. [Google Scholar] [CrossRef]
- Etenko, O.A.; Ivankina, N.F.; Anikina, E.A. Fatty acid composition of bracken fern fronds from the southern and northern zones of Amur Oblast. In Botanical Investigation in Priamurie and Borderline Territory, Proceedings of the Region Conference, Blagoveshchensk, Russia, 24–26 May 2004; Nekrasov, E.V., Ed.; ABBGI FEB RAS: Blagoveshchensk, Russia, 2005; pp. 121–122. (In Russian) [Google Scholar]
- Shalisko, I.V.; Dmitrichenko, M.I.; Pelenko, V.V.; Shakhov, S.V. Changes in consumer properties of bracken using different storage methods. Proceed. Voronezh State Univ. Eng. Tech. 2016, 3, 151–158. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Pumprova, K.; Antoninova, Z.; Rezek, J.; Haisel, D.; Ekrt, L.; Vanek, T.; Langhansova, L. Nutritional and antioxidant potential of fiddleheads from European ferns. Foods 2021, 10, 460. [Google Scholar] [CrossRef]
- Bushway, A.A.; Serreze, D.V.; McGann, D.F.; True, R.H.; Work, T.M.; Bushway, R.J. Effect of processing method and storage time on the nutrient composition of fiddlehead greens. J. Food Sci. 1985, 50, 1491–1492. [Google Scholar] [CrossRef]
- DeLong, J.M.; Hodges, D.M.; Prange, R.K.; Forney, C.F.; Fan, L.; Bishop, M.C.; Elliot, M.L.; Jordan, M.A.; Doucette, C. The influence of cold water storage on fatty acids, antioxidant content and activity, and microbial load in ostrich fern (Matteuccia struthiopteris) fiddleheads. Can. J. Plant Sci. 2013, 93, 683–697. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Duncan, H.J. Distribution of glycolipids and phospholipids in Pteridium aquilinum. Phytochemistry 1974, 13, 979–981. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem. 2007, 101, 1694–1700. [Google Scholar] [CrossRef]
- Haak, L.; Sioen, I.; Raes, K.; Van Camp, J.; De Smet, S. Effect of pan-frying in different culinary fats on the fatty acid profile of pork. Food Chem. 2007, 102, 857–864. [Google Scholar] [CrossRef]
- Ansorena, D.; Guembe, A.; Mendizabal, T.; Astiasaran, I. Effect of fish and oil nature on frying process and nutritional product quality. J. Food Sci. 2010, 75, H62–H67. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.; Quek, S.Y.; Eyres, L. Effect of cooking method on the fatty acid profile of New Zealand King Salmon (Oncorhynchus tshawytscha). Food Chem. 2010, 119, 785–790. [Google Scholar] [CrossRef]
- Neff, M.R.; Bhavsar, S.P.; Braekevelt, E.; Arts, M.T. Effects of different cooking methods on fatty acid profiles in four freshwater fishes from the Laurentian Great Lakes region. Food Chem. 2014, 164, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Galano, J.M.; Durand, T.; Lee, J.C.Y. Profiling of omega-polyunsaturated fatty acids and their oxidized products in salmon after different cooking methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef]
- Tsai, P.J.; Huang, W.C.; Lin, S.W.; Chen, S.N.; Shen, H.J.; Chang, H.; Chuang, L.T. Juniperonic acid incorporation into the phospholipids of murine macrophage cells modulates pro-inflammatory mediator production. Inflammation 2018, 41, 1200–1214. [Google Scholar] [CrossRef]
- Droulez, V.; William, P.G.; Levy, G.; Stobaus, T.; Sinclair, A. Composition of Australian red meat 2002. 2. Fatty acid profile. Food Australia 2006, 58, 335–341. [Google Scholar]
- Kouba, M.; Benatmane, F.; Blochet, J.E.; Mourot, J. Effect of a linseed diet on lipid oxidation, fatty acid composition of muscle, perirenal fat, and raw and cooked rabbit meat. Meat Sci. 2008, 80, 829–834. [Google Scholar] [CrossRef]
- Dugan, M.E.; Vahmani, P.; Turner, T.D.; Mapiye, C.; Juarez, M.; Prieto, N.; Beaulieu, A.D.; Zijlstra, R.T.; Patience, J.F.; Aalhus, J.L. Pork as a source of omega-3 (n-3) fatty acids. J. Clin. Med. 2015, 4, 1999–2011. [Google Scholar] [CrossRef]
- Ye, Y.; Eyres, G.T.; Reis, M.G.; Schreurs, N.M.; Silcock, P.; Agnew, M.P.; Johnson, P.L.; Maclean, P.; Realini, C.E. Fatty acid composition and volatile profile of M. longissimus thoracis from commercial lambs reared in different forage systems. Foods 2020, 9, 1885. [Google Scholar] [CrossRef]
- De Souza Vilela, J.; Alvarenga, T.I.R.C.; Andrew, N.R.; McPhee, M.; Kolakshyapati, M.; Hopkins, D.L.; Ruhnke, I. Technological quality, amino acid and fatty acid profile of broiler meat enhanced by dietary inclusion of black soldier fly larvae. Foods 2021, 10, 297. [Google Scholar] [CrossRef]
- Czerner, M.; Agustinelli, S.P.; Guccione, S.; Yeannes, M.I. Effect of different preservation processes on chemical composition and fatty acid profile of anchovy (Engraulis anchoita). Int. J. Food Sci. Nutr. 2015, 66, 887–894. [Google Scholar] [CrossRef]
- Boulom, S.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Seasonal changes in lipid, fatty acid, α-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds, New Zealand. Food Chem. 2014, 161, 261–269. [Google Scholar] [CrossRef]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbo, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef] [Green Version]
- Wolff, R.L.; Christie, W.W. Structures, practical sources (gymnosperm seeds), gas-liquid chromatographic data (equivalent chain lengths), and mass spectrometric characteristics of all-cis Δ5-olefinic acids. Eur. J. Lipid Sci. Tech. 2002, 104, 234–244. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Aitzetmuller, K. Unusual Δ5cis-fatty acids in seed oils of Cimicifuga species. J. High Res. Chrom. 1997, 20, 237–241. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Tallon, S.J.; Vyssotski, M.V.; Catchpole, O.J. Extraction of lipids from New Zealand fern fronds using near-critical dimethyl ether and dimethyl ether–water–ethanol mixtures. J. Supercrit. Fluids 2021, 170, 105137. [Google Scholar] [CrossRef]
| Pteridium aquilinum | Matteuccia struthiopteris | Osmundastrum asiaticum | ||||
|---|---|---|---|---|---|---|
| Primorsky Krai | Amur Oblast | Primorsky Krai | Amur Oblast | Primorsky Krai | Amur Oblast | |
| Total lipids, % wet weight | ||||||
| 1.02 ± 0.07 | 1.23 ± 0.10 | 0.85 ± 0.12 | 0.99 ± 0.14 | 0.80 ± 0.09 | 0.92 ± 0.06 | |
| Fatty acid, % of sum | ||||||
| 14:0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 14:1n-5 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 15:0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 16:0 | 26.3 ± 0.9 | 25.0 ± 0.8 | 26.6 ± 0.6 | 26.6 ± 1.0 | 30.0 ± 0.3 | 25.3 ± 0.5 |
| 16:1n-9 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 16:1n-7 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| t-16:1n-13 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| 16:2n-6 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 |
| 16:3n-3 | 1.7 ± 0.2 | 1.9 ± 0.6 | 1.3 ± 0.1 | 1.7 ± 0.4 | 2.5 ± 0.3 | 2.8 ± 0.2 |
| 17:0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 18:0 | 1.7 ± 0.0 | 2.0 ± 0.2 | 1.0 ± 0.0 | 1.5 ± 0.1 | 1.6 ± 0.5 | 1.2 ± 0.0 |
| 18:1n-9 | 5.0 ± 0.2 | 5.9 ± 0.4 | 5.5 ± 0.3 | 10.0 ± 0.1 | 5.9 ± 0.5 | 5.8 ± 0.2 |
| 18:1n-7 | 0.6 ± 0.0 | 0.9 ± 0.2 | 1.5 ± 0.0 | 1.6 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.3 |
| 18:1n-5 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 |
| 18:2n-6 | 27.0 ± 0.4 | 25.1 ± 0.8 | 20.9 ± 0.5 | 19.9 ± 0.7 | 18.0 ± 1.6 | 17.0 ± 1.0 |
| 18:3n-6 | 0.9 ± 0.0 | 2.5 ± 0.1 | 1.7 ± 0.1 | 2.7 ± 0.1 | 2.2 ± 0.3 | 2.4 ± 0.1 |
| 18:3n-3 | 10.8 ± 0.6 | 12.8 ± 1.3 | 16.9 ± 0.1 | 14.4 ± 1.1 | 22.0 ± 1.3 | 24.0 ± 1.2 |
| 18:4n-3 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| 20:0 | 1.4 ± 0.1 | 1.5 ± 0.2 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.2 | 0.4 ± 0.0 |
| 20:1n-9 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:2n-6 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 5,11,14-20:3 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.1 |
| 20:3n-6 | 1.3 ± 0.1 | 1.5 ± 0.4 | 2.6 ± 0.2 | 1.8 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.1 |
| 20:4n-6 (ARA) | 13.5 ± 0.4 | 11.8 ± 0.4 | 12.8 ± 0.5 | 9.3 ± 0.1 | 6.4 ± 0.5 | 8.8 ± 0.2 |
| 5,11,14,17-20:4 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 |
| 20:4n-3 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 |
| 20:5n-3 (EPA) | 0.8 ± 0.0 | 1.0 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.9 ± 0.2 | 3.2 ± 0.3 |
| 22:0 | 2.5 ± 0.4 | 2.1 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.0 |
| 23:0 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.0 | n.d. |
| 24:0 | 3.2 ± 0.7 | 2.7 ± 0.2 | 1.5 ± 0.3 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.0 |
| 26:0 | 0.6 ± 0.2 | 0.5 ± 0.0 | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Others 1 | 0.8 ± 0.1 | 0.7 ± 0.0 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 |
| SFAs | 36.5 ± 0.7 | 34.5 ± 1.6 | 31.3 ± 0.9 | 32.2 ± 1.2 | 35.1 ± 0.5 | 30.0 ± 0.6 |
| MUFAs | 6.4 ± 0.1 | 7.8 ± 0.3 | 9.1 ± 0.3 | 14.6 ± 0.2 | 8.1 ± 0.4 | 7.9 ± 0.2 |
| PUFAs | 57.1 ± 0.6 | 57.7 ± 1.3 | 59.6 ± 1.0 | 53.3 ± 1.4 | 56.8 ± 0.9 | 62.1 ± 0.8 |
| n-6 | 43.5 ± 0.3 | 41.5 ± 0.8 | 38.7 ± 1.1 | 34.3 ± 0.5 | 28.3 ± 0.8 | 30.6 ± 1.4 |
| n-3 | 13.6 ± 0.8 | 16.1 ± 1.9 | 20.9 ± 0.2 | 18.9 ± 1.8 | 28.5 ± 1.6 | 31.4 ± 1.5 |
| ARA/EPA | 17.0 | 11.3 | 5.6 | 3.9 | 2.2 | 2.8 |
| (n-6)/(n-3) | 3.2 | 2.6 | 1.8 | 1.8 | 1.0 | 1.0 |
| Raw | Dried | Salted 1 | Salted 2 | Raw | Dried | Salted 1 | Salted 2 | |
|---|---|---|---|---|---|---|---|---|
| Total lipids | ||||||||
| % wet weight | 1.23 ± 0.10 a,b | 6.88 ± 0.84 c | 0.98 ± 0.03 d | 1.07 ± 0.07 b,d | ||||
| % dry weight | 8.43 ± 0.17 a | 7.65 ± 0.96 a,b | 7.90 ± 0.25 b | 7.84 ± 0.52 a,b | ||||
| Fatty acid | % of sum | mg/g dry weight | ||||||
| 14:0 | 0.3 ± 0.0 a | 0.4 ± 0.1 a,c | 0.5 ± 0.1 b,c | 0.5 ± 0.1 b,c | 0.15 ± 0.03 a | 0.13 ± 0.05 a | 0.20 ± 0.06 a | 0.19 ± 0.03 a |
| 16:0 | 24.3 ± 0.6 a | 27.4 ± 0.9 b | 36.2 ± 0.2 c | 37.1 ± 0.5 c | 11.07 ± 0.38 a,b | 9.25 ± 1.78 b | 15.08 ± 2.22 a,c | 12.87 ± 0.49 b,c |
| 16:1n-9 | 0.15 ± 0.02 a,b | 0.12 ± 0.01 b | 0.16 ± 0.00 a | 0.17 ± 0.00 a | 0.07 ± 0.01 a | 0.04 ± 0.01 b | 0.07 ± 0.01 a | 0.06 ± 0.00 a |
| 16:1n-7 | 0.3 ± 0.1 a | 0.2 ± 0.0 a | 0.4 ± 0.0 a | 0.3 ± 0.0 a | 0.12 ± 0.02 a | 0.09 ± 0.03 a | 0.15 ± 0.02 a | 0.12 ± 0.01 a |
| t-16:1n-13 | 0.16 ± 0.04 a | 0.16 ± 0.03 a | 0.40 ± 0.01 b | 0.37 ± 0.04 b | 0.07 ± 0.02 a | 0.06 ± 0.02 a | 0.17 ± 0.03 b | 0.13 ± 0.01 b |
| 16:3n-3 | 2.0 ± 0.5 a,b | 1.4 ± 0.1 b | 1.0 ± 0.0 a | 1.0 ± 0.1 a | 0.92 ± 0.27 a | 0.49 ± 0.11 a | 0.44 ± 0.07 a | 0.36 ± 0.03 a |
| 18:0 | 1.9 ± 0.1 a | 2.1 ± 0.0 a | 2.5 ± 0.1 b | 2.7 ± 0.1 c | 0.88 ± 0.03 a,b | 0.70 ± 0.11 a | 1.06 ± 0.19 a,b | 0.94 ± 0.05 b |
| 18:1n-9 | 5.8 ± 0.5 a | 4.4 ± 0.6 b | 7.4 ± 0.1 c | 7.2 ± 0.2 c | 2.64 ± 0.35 a | 1.51 ± 0.32 b | 3.08 ± 0.52 a | 2.51 ± 0.14 a |
| 18:1n-7 | 0.9 ± 0.2 a,c | 0.6 ± 0.0 b | 0.9 ± 0.0 c | 1.1 ± 0.1 c | 0.39 ± 0.06 a | 0.21 ± 0.05 b | 0.38 ± 0.05 a | 0.37 ± 0.03 a |
| 18:2n-6 | 25.4 ± 0.9 a | 24.4 ± 0.8 a | 19.2 ± 0.3 b | 21.2 ± 0.3 c | 11.59 ± 0.89 a | 8.30 ± 1.33 b | 8.02 ± 1.16 b | 7.33 ± 0.16 b |
| 18:3n-6 | 2.6 ± 0.2 a | 1.9 ± 0.2 b | 1.6 ± 0.0 b | 1.5 ± 0.0 b | 1.16 ± 0.02 a | 0.64 ± 0.15 b | 0.66 ± 0.10 b | 0.53 ± 0.02 b |
| 18:3n-3 | 13.1 ± 1.0 a | 11.4 ± 0.2 a | 7.3 ± 0.1 b | 6.6 ± 0.4 b | 5.99 ± 0.73 a | 3.91 ± 0.66 b | 3.04 ± 0.49 b | 2.30 ± 0.05 b |
| 18:4n-3 | 0.20 ± 0.02 a | 0.15 ± 0.01 b | 0.11 ± 0.00 c | 0.10 ± 0.00 c | 0.09 ± 0.01 a | 0.05 ± 0.01 b | 0.04 ± 0.01 b | 0.04 ± 0.00 b |
| 20:0 | 1.4 ± 0.2 a | 1.4 ± 0.1 a | 1.6 ± 0.2 a | 1.5 ± 0.0 a | 0.63 ± 0.10 a | 0.48 ± 0.10 a | 0.67 ± 0.16 a | 0.53 ± 0.02 a |
| 20:1n-9 | 0.13 ± 0.03 a,b,c | 0.14 ± 0.02 a,b | 0.18 ± 0.01 a,c | 0.18 ± 0.02 a,b,c | 0.06 ± 0.02 a | 0.05 ± 0.01 a | 0.08 ± 0.02 a | 0.06 ± 0.01 a |
| 20:2n-6 | 0.13 ± 0.00 a | 0.37 ± 0.02 b | 0.25 ± 0.01 c | 0.23 ± 0.02 c | 0.06 ± 0.00 a | 0.12 ± 0.03 a | 0.10 ± 0.02 a | 0.08 ± 0.01 a |
| 5,11,14-20:3 | 0.35 ± 0.06 a | 0.55 ± 0.07 b | 0.37 ± 0.02 a | 0.35 ± 0.06 a | 0.16 ± 0.03 a | 0.18 ± 0.04 a | 0.15 ± 0.01 a | 0.12 ± 0.02 a |
| 20:3n-6 | 1.6 ± 0.5 a,b | 1.7 ± 0.0 a | 2.4 ± 0.1 b | 1.7 ± 0.1 a | 0.74 ± 0.23 a,b | 0.60 ± 0.12 a | 1.00 ± 0.11 b | 0.59 ± 0.04 a |
| 20:4n-6 (ARA) | 12.1 ± 0.7 a | 13.7 ± 1.4 a | 9.2 ± 0.2 b | 8.2 ± 0.2 c | 5.51 ± 0.07 a | 4.63 ± 0.77 a,b | 3.85 ± 0.51 b | 2.83 ± 0.08 b |
| 5,11,14,17-20:4 | 0.09 ± 0.02 a | 0.08 ± 0.01 a | 0.14 ± 0.01 b | 0.07 ± 0.01 a | 0.04 ± 0.01 a,b | 0.03 ± 0.01 a | 0.06 ± 0.01 b | 0.02 ± 0.00 a |
| 20:4n-3 | 0.12 ± 0.02 a | 0.12 ± 0.01 a | 0.16 ± 0.01 b | 0.10 ± 0.00 a | 0.05 ± 0.01 a | 0.04 ± 0.01 a | 0.07 ± 0.01 a | 0.03 ± 0.00 a |
| 20:5n-3 (EPA) | 1.1 ± 0.2 a | 0.9 ± 0.1 a | 0.6 ± 0.0 b | 0.5 ± 0.0 c | 0.49 ± 0.07 a | 0.32 ± 0.05 b | 0.26 ± 0.04 b,c | 0.17 ± 0.00 c |
| 22:0 | 2.0 ± 0.1 a | 2.2 ± 0.1 a,b | 2.2 ± 0.2 a,b | 2.3 ± 0.1 b | 0.91 ± 0.07 a | 0.74 ± 0.14 a | 0.92 ± 0.20 a | 0.78 ± 0.02 a |
| 24:0 | 2.7 ± 0.3 a | 3.0 ± 0.3 a,b | 3.5 ± 0.3 b | 3.3 ± 0.1 a,b | 1.22 ± 0.17 a | 1.00 ± 0.17 a | 1.46 ± 0.32 a | 1.15 ± 0.05 a |
| 26:0 | 0.5 ± 0.0 a | 0.6 ± 0.1 a | 0.8 ± 0.0 b | 0.7 ± 0.0 a | 0.23 ± 0.03 a | 0.20 ± 0.02 a | 0.32 ± 0.06 a | 0.23 ± 0.01 a |
| Others 1 | 0.7 ± 0.1 a | 0.6 ± 0.0 a | 1.0 ± 0.0 b | 0.9 ± 0.1 b | 0.31 ± 0.06 a,b | 0.21 ± 0.03 a | 0.42 ± 0.07 b | 0.33 ± 0.04 b |
| SFAs | 34.8 ± 1.2 a | 38.1 ± 1.1 b | 48.1 ± 0.7 c | 49.0 ± 0.5 c | 16.07 ± 0.83 a,b | 13.03 ± 2.42 a | 20.23 ± 3.27 b | 17.10 ± 0.66 a,b |
| MUFAs | 7.6 ± 0.4 a | 5.9 ± 0.7 b | 9.9 ± 0.1 c | 9.9 ± 0.2 c | 3.48 ± 0.36 a | 2.03 ± 0.42 b | 4.13 ± 0.67 a | 3.44 ± 0.17 a |
| PUFAs | 57.6 ± 1.3 a | 56.0 ± 0.7 a | 42.0 ± 0.8 b | 41.1 ± 0.5 b | 26.03 ± 1.55 a | 18.91 ± 2.96 b | 17.38 ± 2.48 b | 14.13 ± 0.24 b |
| n-6 | 42.3 ± 1.4 a | 42.7 ± 0.8 a | 33.1 ± 0.6 b | 33.2 ± 0.3 b | 19.28 ± 1.10 a | 14.49 ± 2.23 b | 13.81 ± 1.91 b | 11.50 ± 0.29 b |
| n-3 | 15.3 ± 1.0 a | 13.3 ± 0.8 a | 8.8 ± 0.4 b | 7.9 ± 0.2 c | 6.75 ± 0.74 a | 4.42 ± 0.74 b | 3.57 ± 0.56 b | 2.63 ± 0.05 b |
| Total | 45.58 ± 2.69 a | 33.97 ± 5.69 a,b | 41.74 ± 6.41 a,b | 34.67 ± 1.05 b | ||||
| ARA/EPA | 11.2 | 14.8 | 14.7 | 16.7 | ||||
| (n-6)/(n-3) | 2.8 | 3.2 | 3.8 | 4.2 | ||||
| Raw | Dried | Raw | Dried | |
|---|---|---|---|---|
| Total lipids | ||||
| % wet weight | 0.99 ± 0.14 a | 7.88 ± 0.45 b | ||
| % dry weight | 7.64 ± 1.16 a | 8.84 ± 0.49 a | ||
| Fatty acid | % of sum | mg/g dry weight | ||
| 14:0 | 0.31 ± 0.01 a | 0.31 ± 0.03 a | 0.12 ± 0.02 a | 0.12 ± 0.01 a |
| 16:0 | 23.8 ± 0.1 a | 24.6 ± 0.8 a | 9.05 ± 1.17 a | 9.39 ± 0.41 a |
| 16:1n-9 | 0.69 ± 0.02 a | 0.58 ± 0.06 a | 0.26 ± 0.04 a | 0.22 ± 0.03 a |
| 16:1n-7 | 1.03 ± 0.03 a | 0.83 ± 0.06 b | 0.39 ± 0.05 a | 0.32 ± 0.03 a |
| t-16:1n-13 | 0.28 ± 0.13 a | 0.18 ± 0.06 a | 0.11 ± 0.07 a | 0.07 ± 0.03 a |
| 16:3n-3 | 1.89 ± 0.51 a | 1.87 ± 0.26 a | 0.74 ± 0.29 a | 0.72 ± 0.12 a |
| 18:0 | 1.39 ± 0.06 a | 1.21 ± 0.05 b | 0.53 ± 0.08 a | 0.46 ± 0.03 a |
| 18:1n-9 | 9.2 ± 0.1 a | 6.1 ± 0.4 b | 3.49 ± 0.49 a | 2.34 ± 0.22 b |
| 18:1n-7 | 1.41 ± 0.16 a | 1.10 ± 0.21 a | 0.53 ± 0.05 a | 0.42 ± 0.07 a |
| 18:2n-6 | 20.4 ± 1.0 a | 19.5 ± 0.5 a | 7.72 ± 0.62 a | 7.45 ± 0.16 a |
| 18:3n-6 | 2.9 ± 0.0 a | 2.3 ± 0.5 a | 1.12 ± 0.15 a | 0.87 ± 0.21 a |
| 18:3n-3 | 15.9 ± 0.8 a | 19.2 ± 0.4 b | 6.08 ± 1.07 a | 7.32 ± 0.44 a |
| 18:4n-3 | 0.20 ± 0.01 a | 0.17 ± 0.04 a | 0.08 ± 0.01 a | 0.07 ± 0.02 a |
| 20:0 | 0.40 ± 0.04 a | 0.38 ± 0.03 a | 0.15 ± 0.02 a | 0.15 ± 0.01 a |
| 20:1n-9 | 0.23 ± 0.02 a | 0.23 ± 0.02 a | 0.09 ± 0.02 a | 0.09 ± 0.01 a |
| 20:2n-6 | 0.15 ± 0.03 a | 0.35 ± 0.03 b | 0.06 ± 0.00 a | 0.13 ± 0.01 b |
| 5,11,14-20:3 | 0.20 ± 0.02 a | 0.32 ± 0.02 b | 0.07 ± 0.01 a | 0.12 ± 0.00 b |
| 20:3n-6 | 1.99 ± 0.1 a | 2.44 ± 0.3 a | 0.75 ± 0.06 a | 0.93 ± 0.08 b |
| 20:4n-6 (ARA) | 10.7 ± 0.6 a | 11.4 ± 0.5 a | 4.06 ± 0.30 a | 4.36 ± 0.02 a |
| 5,11,14,17-20:4 | 0.20 ± 0.01 a | 0.23 ± 0.02 a | 0.08 ± 0.01 a | 0.09 ± 0.01 a |
| 20:4n-3 | 0.17 ± 0.01 a | 0.17 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.00 a |
| 20:5n-3 (EPA) | 2.9 ± 0.1 a | 2.5 ± 0.3 a | 1.09 ± 0.16 a | 0.94 ± 0.11 a |
| 22:0 | 0.79 ± 0.04 a | 0.74 ± 0.04 a | 0.30 ± 0.03 a | 0.28 ± 0.01 a |
| 24:0 | 1.41 ± 0.07 a | 1.46 ± 0.09 a | 0.54 ± 0.05 a | 0.56 ± 0.03 a |
| 26:0 | 0.45 ± 0.03 a | 0.39 ± 0.04 a | 0.17 ± 0.03 a | 0.15 ± 0.01 a |
| Others 1 | 1.0 ± 0.1 a | 1.4 ± 0.1 b | 0.40 ± 0.09 a | 0.55 ± 0.08 a |
| SFAs | 29.8 ± 0.9 a | 30.6 ± 1.4 a | 11.65 ± 1.68 a | 11.92 ± 0.53 a |
| MUFAs | 13.2 ± 0.3 a | 9.7 ± 0.4 b | 5.03 ± 0.75 a | 3.72 ± 0.27 a |
| PUFAs | 57.0 ± 0.8 a | 59.7 ± 1.2 b | 21.36 ± 2.41 a | 22.54 ± 0.81 a |
| n-6 | 36.7 ± 1.6 a | 36.6 ± 0.9 a | 13.89 ± 1.14 a | 13.96 ± 0.30 a |
| n-3 | 20.3 ± 2.1 a | 23.1 ± 1.6 a | 7.46 ± 1.26 a | 8.58 ± 0.52 a |
| Total | 38.04 ± 4.84 a | 38.19 ± 1.41 a | ||
| ARA/EPA | 3.7 | 4.7 | ||
| (n-6)/(n-3) | 1.8 | 1.6 | ||
| Raw | Dried at 110 °C | Air-Dried | Raw | Dried at 110 °C | Air-Dried | |
|---|---|---|---|---|---|---|
| Total lipids | ||||||
| % wet weight | 0.92 ± 0.06 a | 1.03 ± 0.06 a | 0.92 ± 0.05 a | |||
| % dry weight | 4.96 ± 0.49 a | 5.50 ± 0.24 a | 4.95 ± 0.58 a | |||
| Fatty acid | % of sum | mg/g dry weight | ||||
| 14:0 | 0.13 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.00 b | 0.03 ± 0.00 b |
| 16:0 | 25.3 ± 0.5 a | 26.0 ± 0.3 a | 25.6 ± 0.4 a | 6.2 ± 0.4 a | 5.9 ± 0.2 a | 5.6 ± 0.4 a |
| 16:1n-9 | 0.13 ± 0.01 a | 0.14 ± 0.00 a | 0.14 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| 16:1n-7 | 0.17 ± 0.03 a | 0.19 ± 0.02 a | 0.17 ± 0.03 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a |
| t-16:1n-13 | 0.06 ± 0.00 a | 0.06 ± 0.02 a | 0.06 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 16:3n-3 | 2.8 ± 0.2 a | 2.8 ± 0.1 a | 2.8 ± 0.1 a | 0.69 ± 0.09 a | 0.62 ± 0.05 a | 0.60 ± 0.07 a |
| 18:0 | 1.16 ± 0.05 a | 1.19 ± 0.05 a | 1.20 ± 0.05 a | 0.28 ± 0.01 a | 0.27 ± 0.02 a | 0.26 ± 0.03 a |
| 18:1n-9 | 5.8 ± 0.2 a | 6.1 ± 0.3 a | 5.9 ± 0.51 a | 1.42 ± 0.15 a | 1.38 ± 0.12 a | 1.29 ± 0.23 a |
| 18:1n-7 | 0.99 ± 0.30 a | 0.99 ± 0.23 a | 1.03 ± 0.32 a | 0.24 ± 0.06 a | 0.22 ± 0.04 a | 0.22 ± 0.05 a |
| 18:2n-6 | 17.0 ± 1.0 a | 17.5 ± 0.6 a | 16.7 ± 0.9 a | 4.1 ± 0.5 a | 3.9 ± 0.2 a | 3.6 ± 0.4 a |
| 18:3n-6 | 2.4 ± 0.1 a | 2.4 ± 0.1 a | 2.4 ± 0.04 a | 0.58 ± 0.06 a | 0.53 ± 0.04 a | 0.52 ± 0.06 a |
| 18:3n-3 | 24.0 ± 1.2 a | 24.2 ± 0.9 a | 24.2 ± 1.1 a | 5.8 ± 0.5 a | 5.5 ± 0.3 a | 5.2 ± 0.5 a |
| 18:4n-3 | 0.56 ± 0.05 a | 0.56 ± 0.03 a | 0.57 ± 0.05 a | 0.14 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a |
| 20:0 | 0.35 ± 0.02 a | 0.33 ± 0.03 a | 0.37 ± 0.03 a | 0.09 ± 0.00 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a |
| 20:1n-9 | 0.13 ± 0.03 a | 0.15 ± 0.03 a | 0.16 ± 0.03 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.01 a |
| 20:2n-6 | 0.23 ± 0.02 a | 0.22 ± 0.01 a | 0.24 ± 0.03 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a |
| 5,11,14-20:3 | 0.79 ± 0.07 a | 0.72 ± 0.04 a | 0.78 ± 0.07 a | 0.19 ± 0.02 a | 0.16 ± 0.01 a | 0.17 ± 0.02 a |
| 20:3n-6 | 1.29 ± 0.14 a | 1.18 ± 0.07 a | 1.26 ± 0.13 a | 0.31 ± 0.05 a | 0.27 ± 0.02 a | 0.27 ± 0.05 a |
| 20:4n-6 (ARA) | 8.9 ± 0.2 a | 7.8 ± 0.1 b | 8.6 ± 0.2 a | 2.15 ± 0.21 a | 1.76 ± 0.07 a | 1.86 ± 0.18 a |
| 5,11,14,17-20:4 | 0.47 ± 0.01 a | 0.46 ± 0.01 a | 0.49 ± 0.04 a | 0.12 ± 0.01 a | 0.10 ± 0.01 a | 0.11 ± 0.02 a |
| 20:4n-3 | 0.31 ± 0.01 a | 0.29 ± 0.01 a | 0.29 ± 0.01 a | 0.08 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a |
| 20:5n-3 (EPA) | 3.2 ± 0.3 a | 2.9 ± 0.2 a | 3.2 ± 0.3 a | 0.78 ± 0.10 a | 0.66 ± 0.06 a | 0.69 ± 0.09 a |
| 22:0 | 1.17 ± 0.04 a | 1.12 ± 0.08 a | 1.18 ± 0.05 a | 0.29 ± 0.03 a | 0.25 ± 0.02 a | 0.26 ± 0.03 a |
| 24:0 | 1.39 ± 0.03 a | 1.27 ± 0.06 a,b | 1.31 ± 0.04 b | 0.34 ± 0.03 a | 0.29 ± 0.02 b | 0.28 ± 0.02 b |
| 26:0 | 0.29 ± 0.07 a | 0.27 ± 0.08 a | 0.25 ± 0.06 a | 0.07 ± 0.01 a | 0.06 ± 0.02 a | 0.05 ± 0.01 a |
| Others 1 | 1.0 ± 0.1 a | 1.0 ± 0.1 a | 1.0 ± 0.0 a | 0.24 ± 0.00 a | 0.23 ± 0.02 a | 0.23 ± 0.02 a |
| SFAs | 31.8 ± 2.0 a | 33.2 ± 0.4 a | 32.9 ± 0.4 a | 8.0 ± 0.5 a | 7.5 ± 0.2 a | 7.1 ± 0.6 a |
| MUFAs | 7.9 ± 0.2 a | 8.3 ± 0.1 a | 8.1 ± 0.2 a | 1.9 ± 0.1 a | 1.9 ± 0.1 a | 1.8 ± 0.2 a |
| PUFAs | 60.4 ± 2.2 a | 58.6 ± 0.4 a | 59.0 ± 0.3 a | 14.5 ± 1.2 a | 13.2 ± 0.6 a | 12.8 ± 1.2 a |
| n-6 | 30.7 ± 1.4 a | 29.9 ± 0.7 a | 30.1 ± 1.2 a | 7.5 ± 0.8 a | 6.7 ± 0.3 a | 6.5 ± 0.7 a |
| n-3 | 29.7 ± 1.9 a | 28.7 ± 0.9 a | 29.0 ± 1.4 a | 7.0 ± 0.6 a | 6.5 ± 0.4 a | 6.3 ± 0.6 a |
| Total | 24.4 ± 1.8 a | 22.5 ± 0.9 a | 21.7 ± 2.0 a | |||
| ARA/EPA | 2.8 | 2.7 | 2.7 | |||
| (n-6)/(n-3) | 1.0 | 1.0 | 1.0 | |||
| Bracken Fern (Salad) | Osmunda (Appetizer) | |||
|---|---|---|---|---|
| Total lipids | ||||
| % wet weight | 2.34 ± 0.27 §# | 0.98 ± 0.27 | ||
| % dry weight | 14.18 ± 1.61 §# | 3.94 ± 1.07 | ||
| Fatty acid | % of sum | mg/g dry weight | % of sum | mg/g dry weight |
| 14:0 | 0.19 ± 0.01 §# | 0.19 ± 0.02 | 0.17 ± 0.02 * | 0.030 ± 0.007 |
| 16:0 | 14. 5 ± 0.9 §# | 14.7 ± 0.9 | 29.6 ± 5.6 | 5.33 ± 0.72 |
| 16:1n-9 | 0.07 ± 0.01 §# | 0.07 ± 0.00 # | 0.09 ± 0.01 * | 0.016 ± 0.003 * |
| 16:1n-7 | 0.18 ± 0.00 # | 0.18 ± 0.02 # | 0.14 ± 0.01 | 0.025 ± 0.007 * |
| t-16:1n-13 | 0.08 ± 0.02 # | 0.08 ± 0.02 §# | 0.07 ± 0.02 | 0.012 ± 0.001 * |
| 16:3n-3 | 0.39 ± 0.02 §# | 0.39 ± 0.04 | 0.43 ± 0.06 * | 0.079 ± 0.019 * |
| 18:0 | 3.05 ± 0.05 §# | 3.12 ± 0.42 §# | 3.11 ± 0.13 * | 0.589 ± 0.229 |
| 18:1n-9 | 20.5 ± 0.6 §# | 21.0 ± 3.2§ # | 13.0 ± 1.5 * | 2.51 ± 1.18 |
| 18:1n-7 | 0.81 ± 0.03 # | 0.83 ± 0.13 §# | 1.33 ± 0.03 | 0.250 ± 0.091 |
| 18:2n-6 | 47.3 ± 1.1 §# | 48.4 ± 6.9 §# | 34.5 ± 5.4 * | 6.71 ± 3.47 |
| 18:3n-6 | 0.75 ± 0.07 §# | 0.76 ± 0.08 # | 0.49 ± 0.03 * | 0.093 ± 0.030 * |
| 18:3n-3 | 3.10 ± 0.12 §# | 3.16 ± 0.34 # | 9.7 ± 0.1 * | 1.81 ± 0.64 * |
| 18:4n-3 | 0.05 ± 0.01 §# | 0.05 ± 0.01 | 0.12 ± 0.03 * | 0.021 ± 0.002 * |
| 20:0 | 0.55 ± 0.01 §# | 0.56 ± 0.06 | 0.44 ± 0.05 | 0.081 ± 0.018 |
| 20:1n-9 | 0.17 ± 0.00 | 0.17 ± 0.02 §# | 0.32 ± 0.04 * | 0.059 ± 0.013 |
| 20:2n-6 | 0.08 ± 0.01 §# | 0.08 ± 0.01 | 0.55 ± 0.13 * | 0.097 ± 0.009 * |
| 5,11,14-20:3 | 0.14 ± 0.04 §# | 0.14 ± 0.02 | 0.27 ± 0.05 * | 0.049 ± 0.010 * |
| 20:3n-6 | 0.71 ± 0.09 # | 0.72 ± 0.03 §# | 0.60 ± 0.12* | 0.110 ± 0.027 * |
| 20:4n-6 (ARA) | 4.3 ± 0.3 §# | 4.4 ± 0.3 # | 1.55 ± 0.21 * | 0.286 ± 0.075 * |
| 5,11,14,17-20:4 | 0.04 ± 0.01 §# | 0.04 ± 0.01 § | 0.14 ± 0.03 * | 0.026 ± 0.005 * |
| 20:4n-3 | 0.05 ± 0.01 §# | 0.05 ± 0.01 # | 0.18 ± 0.03 * | 0.033 ± 0.009 * |
| 20:5n-3 (EPA) | 0.30 ± 0.01 §# | 0.30 ± 0.03 # | 0.71 ± 0.10 * | 0.130 ± 0.031 * |
| 22:0 | 1.15 ± 0.03 §# | 1.17 ± 0.11 # | 0.81 ± 0.13 * | 0.146 ± 0.025 * |
| 24:0 | 1.11 ± 0.10 §# | 1.13 ± 0.04 | 0.92 ± 0.28 | 0.161 ± 0.007 * |
| 26:0 | 0.21 ± 0.03 §# | 0.21 ± 0.01 | 0.13 ± 0.04 * | 0.023 ± 0.002 * |
| Others 1 | 0.30 ± 0.01 §# | 0.30 ± 0.03 | 0.61 ± 0.09 * | 0.110 ± 0.021 * |
| SFAs | 21.0 ± 1.2 §# | 21.5 ± 1.6 # | 35.7 ± 6.0 | 6.46 ± 1.01 |
| MUFAs | 21.9 ± 0.6 §# | 22.4 ± 3.3 §# | 15.1 ± 1.4 * | 2.90 ± 1.30 |
| PUFAs | 57.1 ± 0.7 # | 58.2 ± 7.6 §# | 49.3 ± 4.7 * | 9.46 ± 4.29 |
| n-6 | 53.3 ± 0.6 §# | 54.5 ± 7.2 §# | 38.0 ± 5.0 | 7.36 ± 3.61 |
| n-3 | 3.7 ± 0.1 §# | 3.7 ± 0.4 # | 11.3 ± 0.4 * | 2.11 ± 0.69 * |
| Total | 102.1 ± 12.4 §# | 18.82 ± 6.58 | ||
| ARA/EPA | 14.4 | 2.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasov, E.V.; Svetashev, V.I. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods 2021, 10, 1220. https://doi.org/10.3390/foods10061220
Nekrasov EV, Svetashev VI. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods. 2021; 10(6):1220. https://doi.org/10.3390/foods10061220
Chicago/Turabian StyleNekrasov, Eduard V., and Vasily I. Svetashev. 2021. "Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids" Foods 10, no. 6: 1220. https://doi.org/10.3390/foods10061220
APA StyleNekrasov, E. V., & Svetashev, V. I. (2021). Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods, 10(6), 1220. https://doi.org/10.3390/foods10061220