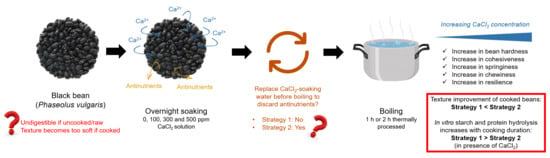
Combined Effects of Calcium Addition and Thermal Processing on the Texture and In Vitro Digestibility of Starch and Protein of Black Beans (Phaseolus vulgaris)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Calcium Uptake during Soaking and Thermal Processing of Black Beans
2.3. Microstructural Evaluation of Cooked Black Beans
2.4. Texture Profile Analysis (TPA) of CaCl2-Soaked and Cooked Black Beans
2.5. Determination of Gelatinisation Properties of CaCl2-Soaked and Cooked Black Beans Using Differential Scanning Calorimetry (DSC)
2.6. Determination of Total Starch Content of CaCl2-Soaked and Cooked Black Beans
2.7. Simulated In Vitro Human Gastric Intestinal Digestion Assay on CaCl2-Soaked and Cooked Black Beans
2.7.1. Preparation of Digestion Solutions
2.7.2. In Vitro Digestibility Procedure
2.7.3. Collection of Digests along the Gastrointestinal Digestion
2.8. Measurement of Starch and Protein Digestibility
2.8.1. Determination of Hydrolysed Starch for CaCl2-Soaked and Cooked Black Beans
2.8.2. Determination of Hydrolysed Protein for Ca-Soaked and Cooked Black Beans
2.9. Statistical Data Analysis
3. Results and Discussion
3.1. Microstructures of Black Beans as Affected by Increasing Thermal Processing Duration
3.2. Effects of Increasing Calcium Concentration and Duration of Thermal Processing on the Textural Properties of Black Beans
3.2.1. Effects of Increasing Cooking Time on the Texture of Black Beans without Receiving Calcium Treatment
3.2.2. Effects of Calcium Addition during Soaking and Cooking on the Texture of Black Beans
3.3. Effects of Calcium Addition and Duration of Thermal Processing on Gelatinisation Properties of Black Beans
3.4. The Effects of Increasing CaCl2 Concentration and Duration of Thermal Processing on the Starch Digestibility of Black Beans
3.4.1. Effects of Increasing Duration of Thermal Processing on the Starch Digestibility of Black Beans without Receiving CaCl2 Treatment
3.4.2. Effects of CaCl2 Addition and Duration of Thermal Processing on the Starch Digestibility of Black Beans
3.5. The Effects of Increasing CaCl2 Concentration and Duration of Thermal Processing on the Protein Digestibility of Black Beans
3.5.1. Effects of Increasing Duration of Thermal Processing on the Protein Digestibility of Black Beans without Receiving CaCl2 Treatment
3.5.2. Effects of CaCl2 Addition and Duration of Thermal Processing on the Protein Digestibility of Black Beans
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Modulating effect of cotyledon cell microstructure on in vitro digestion of starch in legumes. Food Hydrocoll. 2019, 96, 112–122. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Marchie, A.; Kendall, C.W.; Augustin, L.S.; Hamidi, M.; Axelsen, M.; Franceschi, S.; Jenkins, D.J. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallares, A.K.P.; Miranda, B.A.; Truong, N.Q.A.; Kyomugasho, C.; Chigwedere, C.M.; Hendrickx, M.; Grauwet, T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018, 9, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.R.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. Digestion of isolated legume cells in a stomach-duodenum model: Three mechanisms limit starch and protein hydrolysis. Food Funct. 2017, 8, 2573–2582. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Bhattarai, R.R.; Gorham, J.; Gidley, M.J. Intactness of cell wall structure controls the in vitro digestion of starch in legumes. Food Funct. 2016, 7, 1367–1379. [Google Scholar] [CrossRef]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant cell walls: Impact on nutrient bioaccessibility and digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H. Legumes. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 74–79. [Google Scholar]
- Aguilera, J.M.; Stanley, D.W. A review of textural defects in cooked reconstituted legumes? The influence of storage and processing. J. Food Process. Preserv. 1985, 9, 145–169. [Google Scholar] [CrossRef]
- Tovar, J.; Granfeldt, Y.; Bjoerck, I.M. Effect of processing on blood glucose and insulin responses to starch in legumes. J. Agric. Food Chem. 1992, 40, 1846–1851. [Google Scholar] [CrossRef]
- Han, I.H.; Swanson, B.G.; Baik, B.K. Protein digestibility of selected legumes treated with ultrasound and high hydrostatic pressure during soaking. J. Cereal Chem. 2007, 84, 518–521. [Google Scholar] [CrossRef]
- Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculate (L.) Walp subsp. unguiculata. LWT Food Sci. Technol. 2013, 51, 455–461. [Google Scholar] [CrossRef]
- Linsberger-Martin, G.; Weiglhofer, K.; Phuong, T.P.T.; Berghofer, E. High hydrostatic pressure influences antinutritional factors and in vitro protein digestibility of split peas and whole white beans. LWT Food Sci. Technol. 2013, 51, 331–336. [Google Scholar] [CrossRef]
- Piecyk, M.; Wołosiak, R.; Druzynska, B.; Worobiej, E. Chemical composition and starch digestibility in flours from Polish processed legume seeds. Food Chem. 2012, 135, 1057–1064. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Walker, A.F.; Kochhar, N. Effect of processing including domestic cooking on nutritional quality of legumes. Proc. Nutr. Soc. 1982, 41, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L.; Siddhuraju, P.; Saura-Calixto, F. Effect of various processing methods on the in vitro starch digestibility and resistant starch content of Indian pulses. J. Agric. Food Chem. 1998, 46, 4667–4674. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.A.B.; Bates, R.P.; Deng, J.C. Influence of soaking and cooking upon the softening and eating quality of black beans (Phaseolus vulgaris). J. Food Sci. 1981, 46, 1716–1720. [Google Scholar] [CrossRef]
- Yasmin, A.; Zeb, A.; Khalil, A.W.; Paracha, G.M.-U.-D.; Khattak, A.B. Effect of processing on anti-nutritional factors of red kidney bean (Phaseolus vulgaris) grains. Food Bioprocess. Technol. 2008, 1, 415–419. [Google Scholar] [CrossRef]
- Garruti, R.D.S.; Bourne, M.C. Effect of storage conditions of dry bean seeds (Phaseolus vulgaris L.) on texture profile parameters after cooking. J. Food Sci. 1985, 50, 1067–1071. [Google Scholar] [CrossRef]
- Würsch, P.; Del Vedovo, S.; Koellreutter, B. Cell structure and starch nature as key determinants of the digestion rate of starch in legume. Am. J. Clin. Nutr. 1986, 43, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Muñoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria×ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bourne, M.; Stone, A.; Wismer, W. Low temperature blanching effects on chemistry, firmness and structure of canned green beans and carrots. J. Food Sci. 1995, 60, 327–333. [Google Scholar] [CrossRef]
- Wang, C.; Chang, K.; Grafton, K. Canning quality evaluation of pinto and navy beans. J. Food Sci. 1988, 53, 772–776. [Google Scholar] [CrossRef]
- Schoeninger, V.; Coelho, S.R.M.; Bassinello, P.Z. Industrial processing of canned beans. Ciência Rural 2017, 47, e20160672. [Google Scholar] [CrossRef] [Green Version]
- Van Buren, J.P. The chemistry of texture in fruits and vegetables. J. Texture Stud. 1979, 10, 1–23. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Glenn, G.M.; Reddy, A.S.N. Calcium and fruit softening: Physiology and biochemistry. Hortic. Rev. 2011, 10, 107–152. [Google Scholar]
- Van Buggenhout, S.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Pectins in processed fruits and vegetables: Part III-texture engineering. Compr. Rev. Food Sci. Food Saf. 2009, 8, 105–117. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R. Canned whole dry beans and bean products. In Dry Beans and Pulses Production, Processing and Nutrition; Siddiq, M., Uebersax, M.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 155–183. [Google Scholar]
- Howard, L.R.; White, B.L.; Uebersax, M.A.; Siddiq, M. Dry Beans Processing, Quality Evaluation, and Nutrition; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 559–587. [Google Scholar]
- Gwala, S.; Wainana, I.; Pallares, A.P.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Texture and interlinked post-process microstructures determine the in vitro starch digestibility of Bambara groundnuts with distinct hard-to-cook levels. Food Res. Int. 2019, 120, 1–11. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some Indian kidney bean (Phaseolus vulgaris L) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Guiné, R.P.F.; Roque, A.R.F.; Seiça, F.F.A.; Batista, C.E.O. Effect of chemical pretreatments on the physical properties of kiwi. ETP Int. J. Food Eng. 2016, 2, 90–95. [Google Scholar] [CrossRef]
- Texture Technologies. Texture Profile Analysis. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 9 March 2020).
- Chigwedere, C.M.; Olaoye, T.F.; Kyomugasho, C.; Kermani, Z.J.; Pallares, A.P.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Mechanistic insight into softening of Canadian wonder common beans (Phaseolus vulgaris) during cooking. Food Res. Int. 2018, 106, 522–531. [Google Scholar] [CrossRef]
- Leong, S.Y.; Du, D.; Oey, I. Pulsed Electric Fields enhances calcium infusion for improving the hardness of blanched carrots. Innov. Food Sci. Emerg. Technol. 2018, 47, 46–55. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrire, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Abduh, S.B.; Leong, S.Y.; Agyei, D.; Oey, I. Understanding the properties of starch in potatoes (Solanum tuberosum var. Agria) after being treated with pulsed electric field processing. Foods 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. In vitro peptic digestion of ovomucin-depleted egg white affected by pH, temperature and pulsed electric fields. Food Chem. 2017, 231, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gwala, S.; Pallares Pallares, A.; Pälchen, K.; Hendrickx, M.; Grauwet, T. In vitro starch and protein digestion kinetics of cooked bambara groundnuts depend on processing intensity and hardness sorting. Food Res. Int. 2020, 137, 109512. [Google Scholar] [CrossRef]
- Miranda, J.; Carvalho, L.; Vieira, A.; Costa, R.; Guimaraes, A. Anatomical characterization and evaluation of starch granules in grains of black common bean and cowpea in raw and cooked. Chem. Eng. Trans. 2015, 44, 85–90. [Google Scholar]
- Njoroge, D.M.; Kinyanjui, P.K.; Chigwedere, C.M.; Christiaens, S.; Makokha, A.O.; Sila, D.N.; Hendrickx, M.E. Mechanistic insight into common bean pectic polysaccharide changes during storage, soaking and thermal treatment in relation to the hard-to-cook defect. Food Res. Int. 2016, 81, 39–49. [Google Scholar] [CrossRef]
- Tovar, J.; Francisco, A.d.; Bjorck, I.; Asp, N.-G. Relationship between microstructure and in vitro digestibility of starch in precooked leguminous seed flours. Food Struct. 1991, 10, 2. [Google Scholar]
- Berg, T.; Singh, J.; Hardacre, A.; Boland, M. The role of cotyledon cell structure during in vitro digestion of starch in navy beans. Carbohydr. Polym. 2012, 87, 1678–1688. [Google Scholar] [CrossRef]
- Trinh, K.T.; Glasgow, S. On the texture profile analysis test. In Chemeca 2012: Quality of Life through Chemical Engineering; Barton, A.C.T., Ed.; Engineers Australia: Barton, Australia, 2012; pp. 749–760. ISBN 978-1-9221-0759-6. [Google Scholar]
- Sánchez-Arteaga, H.; Urías-Silvas, J.; Espinosa-Andrews, H.; García-Márquez, E. Effect of chemical composition and thermal properties on the cooking quality of common beans (Phaseolus vulgaris). CYTA J. Food 2015, 13, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Pallares Pallares, A.; Rousseau, S.; Chigwedere, C.M.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Temperature-pressure-time combinations for the generation of common bean microstructures with different starch susceptibilities to hydrolysis. Food Res. Int. 2018, 106, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Öner, M.D.; Bayram, M. Effect of soaking and ultrasound treatments on texture of chickpea. J. Food Sci. Technol. 2011, 50, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Pallares, A.P.; Loosveldt, B.; Karimi, S.N.; Hendrickx, M.; Grauwet, T. Effect of process-induced common bean hardness on structural properties of in vivo generated boluses and consequences for in vitro starch digestion kinetics. Br. J. Nutr. 2019, 122, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Sandhu, K.S.; Ahlawat, R.; Sharma, S. In vitro starch digestibility, pasting and textural properties of mung bean: Effect of different processing methods. J. Food Sci. Technol. 2013, 52, 1642–1648. [Google Scholar] [CrossRef] [Green Version]
- Massey, L.M., Jr.; Woodams, E. Effect of calcium on the texture profile of irradiated carrots, beets and potatoes. J. Texture Stud. 1973, 4, 242–247. [Google Scholar]
- Sterling, C. Effect of solutes and pH on the structure and firmness of cooked carrot. Int. J. Food Sci. Technol. 2007, 3, 367–371. [Google Scholar] [CrossRef]
- Smout, C.; Sila, D.N.; Vu, T.S.; Van Loey, A.M.; Hendrickx, M.E. Effect of preheating and calcium pre-treatment on pectin structure and thermal texture degradation: A case study on carrots. J. Food Eng. 2005, 67, 419–425. [Google Scholar] [CrossRef]
- Kader, Z.M. Study of some factors affecting water absorption by faba beans during soaking. Food Chem. 1995, 53, 235–238. [Google Scholar] [CrossRef]
- Gurtas, F.S.; Ak, M.M.; Evranuz, E.Ö. Water diffusion coefficients of selected legumes grown in Turkey as affected by temperature and variety. Turk. J. Agric. For. 2001, 25, 297–304. [Google Scholar]
- Xu, S. Development and application of an automatic system for determining seed volume kinetics during soaking. Master’s Thesis, The University of Tennessee, Knoxville, TN, USA, August 2010. [Google Scholar]
- Chung, H.-J.; Liu, Q.; Pauls, K.P.; Fan, M.Z.; Yada, R. In vitro starch digestibility, expected glycemic index and some phys-icochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res. Int. 2008, 41, 869–875. [Google Scholar] [CrossRef]
- Santiago-Ramos, D.; Figueroa-Cárdenas, J.D.D.; Véles-Medina, J.J.; Salazar, R. Physicochemical properties of nixtamalized black bean (Phaseolus vulgaris L.) flours. Food Chem. 2018, 240, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Sosulski, F.W. Composition, structure, functionality, and chemical modification of legume starches: A review. Can. J. Physiol. Pharmacol. 1991, 69, 79–92. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W. Starch characteristics of black bean, chickpea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Osorio-Díaz, P.; Bello-Pérez, L.A.; Sáyago-Ayerdi, S.G.; Benítez-Reyes, M.D.P.; Tovar, J.; Paredes-López, O. Effect of processing and storage time on in vitro digestibility and resistant starch content of two bean (Phaseolus vulgaris L) varieties. J. Sci. Food Agric. 2003, 83, 1283–1288. [Google Scholar] [CrossRef]
- de Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Pissini Machado Reis, S.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Bravo, L. Effect of processing on the non-starch polysaccharides and in vitro starch digestibility of legumes. Food Sci. Technol. Int. 1999, 5, 415–423. [Google Scholar] [CrossRef]
- Faki, H.; Venkatarama, L.; Desikachar, H. Effect of processing on the in vitro digestibility of proteins and carbohydrates in some Indian legumes. Plant. Foods Hum. Nutr. 1984, 34, 127–133. [Google Scholar] [CrossRef]
- Rehman, Z.-U.; Shah, W.H. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Thompson, L.U.; Yoon, J.H. Starch digestibility as affected by polyphenols and phytic acid. J. Food Sci. 1984, 49, 1228–1229. [Google Scholar] [CrossRef]
- Han, I.H.; Baik, B.-K. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem. J. 2006, 83, 428–433. [Google Scholar] [CrossRef]
- Njoumi, S.; Amiot, M.J.; Rochette, I.; Bellagha, S.; Mouquet-Rivier, C. Soaking and cooking modify the alpha-galacto-oligosaccharide and dietary fibre content in five Mediterranean legumes. Int. J. Food Sci. Nutr. 2019, 70, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Valverde, C.; Frías, J.; Valverde, S. Changes in the carbohydrate composition of legumes after soaking and cooking. J. Am. Diet. Assoc. 1993, 93, 547–550. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Nishida, W.; Proença, R.P.D.C. Influence of soaking on the nutritional quality of common beans (Phaseolus vulgaris L.) cooked with or without the soaking water: A review. Int. J. Food Sci. Technol. 2010, 45, 2209–2218. [Google Scholar] [CrossRef]
- Knuckles, B.E.; Betschart, A.A. Effect of phytate and other myo-inositol phosphate esters on α-amylase digestion of starch. J. Food Sci. 1987, 52, 719–721. [Google Scholar] [CrossRef]
- Yoon, J.H.; Thompson, L.U.; Jenkins, D.J. The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am. J. Clin. Nutr. 1983, 38, 835–842. [Google Scholar] [CrossRef]
- Thompson, L.U.; Button, C.L.; Jenkins, D.J. Phytic acid and calcium affect the in vitro rate of navy bean starch digestion and blood glucose response in humans. Am. J. Clin. Nutr. 1987, 46, 467–473. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cavallieri, Â.L.F.; Da Cunha, R.L. Cold-set whey protein gels induced by calcium or sodium salt addition. Int. J. Food Sci. Technol. 2010, 45, 348–357. [Google Scholar] [CrossRef]
- Yang, N.; Luan, J.; Ashton, J.; Gorczyca, E.; Kasapis, S. Effect of calcium chloride on the structure and in vitro hydrolysis of heat induced whey protein and wheat starch composite gels. Food Hydrocoll. 2014, 42, 260–268. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Habiba, R. Changes in anti-nutrients, protein solubility, digestibility, and HCl-extractability of ash and phosphorus in vegetable peas as affected by cooking methods. Food Chem. 2002, 77, 187–192. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Damodaran, S.; German, B. Physicochemical and functional properties of oilseed proteins with emphasis on soy proteins. J. New Protein Foods 1985, 5, 107–179. [Google Scholar]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility-digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Naidoo, T.; Gerrano, A.; Mellem, J. The effect of processing on in vitro protein and starch digestibility and predictive glycaemic index of five Vigna unguiculata (cowpea) cultivars. Ann. Univ. Dunarea De Jos Galati. Fascicle VI. Food Technol. 2017, 41, 31–41. [Google Scholar]
- El-Moniem, G.M.A. Sensory evaluation and in vitro protein digestibility of mung bean as affected by cooking time. J. Sci. Food Agric. 1999, 79, 2025–2028. [Google Scholar] [CrossRef]
- Chau, C.-F.; Cheung, P.C.-K. Effect of various processing methods on antinutrients and in vitro digestibility of protein and starch of two Chinese indigenous legume seeds. J. Agric. Food Chem. 1997, 45, 4773–4776. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Drulyte, D.; Orlien, V. The effect of processing on digestion of legume proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chem. 2019, 286, 557–566. [Google Scholar] [CrossRef]
- Hernández-Infante, M.; Sousa, V.; Montalvo, I.; Tena, E. Impact of microwave heating on hemagglutinins, trypsin inhibitors and protein quality of selected legume seeds. Plant. Foods Hum. Nutr. 1998, 52, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.R.; Fuente, G.; Bressani, R. Interrelationships between storage, soaking time, cooking time, nutritive value and other characteristics of the black bean (Phaseolus vulgaris). J. Food Sci. 1975, 40, 587–591. [Google Scholar] [CrossRef]





| Time of Cooking (h) | Strategy 1 | Strategy 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 ppm CaCl2 | 100 ppm CaCl2 | 300 ppm CaCl2 | 500 ppm CaCl2 | 0 ppm CaCl2 | 100 ppm CaCl2 | 300 ppm CaCl2 | 500 ppm CaCl2 | |
| Hardness (N) | ||||||||
| 0 | 461.67 ± 37.83 aA | 477.06 ± 37.14 aA | 481.82 ± 19.61 aA | 489.96 ± 34.78 aA | 461.67 ± 37.83 aA | 477.06 ± 37.14 aA | 481.82 ± 19.61 aA | 489.96 ± 34.78 aA |
| 1 | 31.56 ± 3.69 eB | 38.76 ± 7.32 deB | 46.82 ± 5.83 cdB | 67.00 ± 7.80 bB | 32.00 ± 5.40 eB | 48.04 ± 6.95 cB | 65.25 ± 8.14 bB | 79.29 ± 4.49 aB |
| 2 | 21.69 ± 5.98 defB | 26.05 ± 5.40 cdeB | 27.09 ± 3.92 cdC | 35.34 ± 5.54 abC | 17.17 ± 3.36 fB | 19.86 ± 2.44 efC | 29.63 ± 2.65 bcC | 39.37 ± 7.49 aC |
| Cohesiveness (ratio) | ||||||||
| 0 | 0.26 ± 0.01 aA | 0.26 ± 0.01 aA | 0.29 ± 0.03 aA | 0.29 ± 0.03 aA | 0.26 ± 0.01 aA | 0.26 ± 0.01 aA | 0.29 ± 0.03 aA | 0.29 ± 0.03 aA |
| 1 | 0.24 ± 0.02 cA | 0.25 ± 0.01 bcA | 0.27 ± 0.03 abA | 0.29 ± 0.02 aA | 0.25 ± 0.02 bcA | 0.26 ± 0.02 abcA | 0.26 ± 0.02 abcA | 0.27 ± 0.02 abcA |
| 2 | 0.21 ± 0.01 cdB | 0.23 ± 0.02 bcdB | 0.24 ± 0.02 abcdB | 0.25 ± 0.02 abcB | 0.21 ± 0.02 dB | 0.25 ± 0.02 abA | 0.26 ± 0.03 aA | 0.26 ± 0.03 aA |
| Springiness (mm) | ||||||||
| 0 | 0.69 ± 0.05 aA | 0.67 ± 0.05 aA | 0.68 ± 0.06 aA | 0.74 ± 0.09 aA | 0.69 ± 0.05 aA | 0.67 ± 0.05 aA | 0.68 ± 0.06 aA | 0.74 ± 0.09 aA |
| 1 | 0.40 ± 0.04 cB | 0.41 ± 0.03 cB | 0.44 ± 0.02 cB | 0.51 ± 0.02 abB | 0.41 ± 0.04 cB | 0.43 ± 0.03 cB | 0.49 ± 0.02 bB | 0.54 ± 0.03 aB |
| 2 | 0.31± 0.02 deC | 0.31 ± 0.04 deC | 0.35 ± 0.02 cdC | 0.41 ± 0.03 abC | 0.31 ± 0.03 eC | 0.32 ± 0.03 deC | 0.39 ± 0.03 bcC | 0.44 ± 0.02 aC |
| Chewiness (J) | ||||||||
| 0 | 78.80 ± 4.37 aA | 84.85 ± 5.77 aA | 85.13 ± 15.83 aA | 89.16 ± 10.46 aA | 78.80 ± 4.37 aA | 84.85 ± 5.77 aA | 85.13 ± 15.83 aA | 89.16 ± 10.46 aA |
| 1 | 2.53 ± 0.59 dB | 4.05 ± 0.80 dB | 5.82 ± 0.92 cB | 9.24 ± 1.55 bB | 2.83 ± 0.71 dB | 5.74 ± 1.10 cB | 8.91 ± 1.52 bB | 12.10 ± 1.27 aB |
| 2 | 1.45 ± 0.49 dB | 2.08 ± 0.72 bcdB | 2.39 ± 0.51 bcB | 3.75 ± 0.76 aB | 1.33 ± 0.36 dB | 1.56 ± 0.35 cdC | 2.80 ± 0.56 bB | 4.43 ± 0.92 aC |
| Resilience | ||||||||
| 0 | 0.17 ± 0.02 aA | 0.17 ± 0.01 aA | 0.18 ± 0.03 aA | 0.17 ± 0.02 aA | 0.17 ± 0.02 aA | 0.17 ± 0.01 aA | 0.18 ± 0.03 aA | 0.17 ± 0.02 aA |
| 1 | 0.08 ± 0.01 bB | 0.09 ± 0.01 abB | 0.10 ± 0.01 aB | 0.10 ± 0.01 aB | 0.09 ± 0.02 abB | 0.10 ± 0.01 abB | 0.10 ± 0.01 aB | 0.10 ± 0.02 aB |
| 2 | 0.07 ± 0.01 cB | 0.08 ± 0.01 bcC | 0.08 ± 0.01 abcC | 0.09 ± 0.01 abcB | 0.08 ± 0.00 cC | 0.09 ± 0.00 abcB | 0.10 ± 0.01 aB | 0.09 ± 0.01 abB |
| CaCl2 (ppm) | To (°C) | Tp (°C) | Tc (°C) | ∆H (J/g) | T Range (°C) |
|---|---|---|---|---|---|
| Peak 1 (Starch gelatinisation) | |||||
| 0 | 67.85 a (67.60–68.10) | 72.14 a (71.98–72.30) | 76.87 a (76.24–77.5) | 0.91 b (0.90–0.92) | 9.02 a (8.64–9.40) |
| 100 | 65.41 a (65.31–65.50) | 71.29 a (71.00–71.57) | 73.76 b (73.23–74.29) | 0.94 b (0.93–0.96) | 8.36 a (7.92–8.79) |
| 300 | 67.58 a (67.05–8.11) | 71.32 a (71.07–71.56) | 74.92 ab (74.34–75.50) | 1.16 a (1.12–1.20) | 7.34 a (7.29–7.39) |
| 500 | 66.97 a (66.23–67.71) | 72.41 a (72.31–72.51) | 74.72 ab (74.65–74.78) | 1.18 a (1.15–1.22) | 7.75 a (7.07–8.42) |
| Peak 2 (Melting of amylose-lipid complex and protein denaturation) | |||||
| 0 | 94.55 ab (94.40–94.70) | 95.67 c (95.64–95.70) | 96.59 b (96.33–96.84) | 0.40 b (0.39–0.41) | 2.04 b (1.93–2.14) |
| 100 | 94.28 b (94.25–94.30) | 95.79 bc (95.77–95.81) | 96.47 b (96.41–96.53) | 0.50 ab (0.44–0.57) | 2.20 b (2.16–2.23) |
| 300 | 95.09 a (94.97–95.20) | 96.62 a (96.48–96.75) | 100.65 a (100.07–101.23) | 0.84 a (0.81–0.86) | 5.57 a (5.10–6.03) |
| 500 | 94.60 ab (94.55–94.65) | 96.18 b (96.13–96.23) | 98.80 a (98.51–99.08) | 0.80 ab (0.68–0.93) | 4.20 a (3.86–4.53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpos, M.; Leong, S.Y.; Oey, I. Combined Effects of Calcium Addition and Thermal Processing on the Texture and In Vitro Digestibility of Starch and Protein of Black Beans (Phaseolus vulgaris). Foods 2021, 10, 1368. https://doi.org/10.3390/foods10061368
Alpos M, Leong SY, Oey I. Combined Effects of Calcium Addition and Thermal Processing on the Texture and In Vitro Digestibility of Starch and Protein of Black Beans (Phaseolus vulgaris). Foods. 2021; 10(6):1368. https://doi.org/10.3390/foods10061368
Chicago/Turabian StyleAlpos, Marbie, Sze Ying Leong, and Indrawati Oey. 2021. "Combined Effects of Calcium Addition and Thermal Processing on the Texture and In Vitro Digestibility of Starch and Protein of Black Beans (Phaseolus vulgaris)" Foods 10, no. 6: 1368. https://doi.org/10.3390/foods10061368
APA StyleAlpos, M., Leong, S. Y., & Oey, I. (2021). Combined Effects of Calcium Addition and Thermal Processing on the Texture and In Vitro Digestibility of Starch and Protein of Black Beans (Phaseolus vulgaris). Foods, 10(6), 1368. https://doi.org/10.3390/foods10061368