Effects of Bacillus Subtilis-Fermented White Sword Bean Extract on Adipogenesis and Lipolysis of 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Proximate Analysis
2.2. Cell Culture and Differentiation
2.3. Cell Viability Assay
2.4. Quantification of Triglyceride Content
2.5. Quantification of Free Glycerol Content
2.6. Quantitative RT-PCR Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of FWSBE on Cell Viability in 3T3-L1 Preadipocytes
3.2. Effects of FWSBE on Triglyceride and Free Glycerol Content
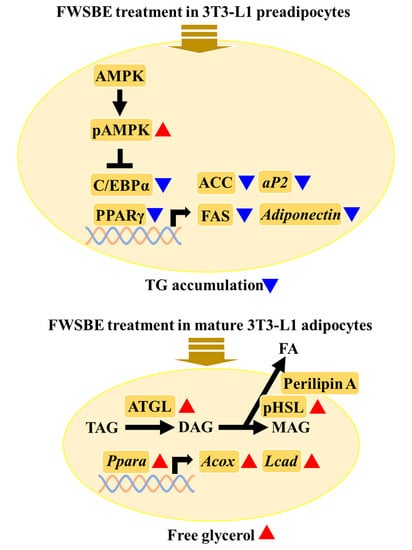
3.3. Effects of FWSBE on the Adipogenesis in 3T3-L1 Pre-Adipocytes
3.4. Effects of FWSBE on Lipolysis in the Mature 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Malnick, S.D.H.; Knobler, H. The medical complications of obesity. J. Assoc. Physicians 2006, 99, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.Y.; Jeon, K.Y.; Jeong, J.W.; Hong, S.H.; Huh, M.K.; Choi, Y.H.; Park, C. Ethanol extracts of Mori folium inhibit adipogenesis through activation of AMPK signaling pathway in 3T3-L1 preadipocytes. J. Life Sci. 2017, 27, 155–163. [Google Scholar] [CrossRef]
- Siersbæk, R.; Nielsen, R.; Mandrup, S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 2012, 23, 56–64. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Choy, L.; Hotamisligil, G.S.; Graves, R.A.; Tontonoz, P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J. Biol. Chem. 1993, 268, 6823–6826. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, H.; Perfield II, J.W.; Obin, M.S.; Greenberg, A.S. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell. Biochem. 2008, 105, 1430–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.-J.; Patel, S.; Miyoshi, H.; Greenberg, A.S.; Kraemer, F.B. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 2009, 50, 2306–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. 2003, 27, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulick, T.; Cresci, S.; Caira, T.; Moore, D.D.; Kelly, D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. USA 1994, 91, 11012–11016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebello, C.J.; Greenway, F.L. Obesity medications in development. Expert Opin. Investig. Drugs 2020, 29, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Elangbam, C.S. Current strategies in the development of anti-obesity drugs and their safety concerns. Vet. Pathol. 2009, 46, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, R.J.; Tschöp, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Models Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Vázquez-Cabral, B.D.; González-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar]
- Li, H.; Kang, J.-H.; Han, J.-M.; Cho, M.-H.; Chung, Y.-J.; Park, K.H.; Shin, D.-H.; Park, H.-Y.; Choi, M.-S.; Jeong, T.-S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, E.-J.; Kim, H.S.; Choi, C.-W.; Choi, S.-W.; Kim, S.-L.; Seo, W.-D.; Do, S.H. Soyasaponin Ab alleviates postmenopausal obesity through browning of white adipose tissue. J. Funct. Foods 2019, 57, 453–464. [Google Scholar] [CrossRef]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab. 2016, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of intestinal inflammation by soybean and soy-derived compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, Y.; Fukumoto, C.; Wada, M.; Yano, E.; Murase, H.; Mizuno, M.; Zaima, N.; Moriyama, T. Validation of antiobesity effects of black soybean seed coat powder suitable as a food material: Comparisons with conventional yellow soybean seed coat powder. Foods 2021, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Pan, M.-H.; Ho, C.-T. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Bae, Y.-I.; Shim, K.-H. Chemical components in different parts of Korean sword bean (Canavalia gladiata). Korean J. Food Preserv. 1999, 6, 475–480. [Google Scholar]
- Gan, R.-Y.; Lui, W.Y.; Corke, H. Sword bean (Canavalia gladiata) as a source of antioxidant phenolics. Int. J. Food Sci. Technol. 2016, 51, 156–162. [Google Scholar] [CrossRef]
- Jeon, K.S.; Na, H.-J.; Kim, Y.-M.; Kwon, H.J. Antiangiogenic activity of 4-O-methylgallic acid from Canavalia gladiata, a dietary legume. Biochem. Biophys. Res. Commun. 2005, 330, 1268–1274. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Seo, K.-I.; Shim, K.-H. Antimicrobial activities of Korean sword bean (Canavalia gladiata) extracts. Korean J. Postharvest Sci. Technol. 2000, 7, 113–116. [Google Scholar]
- Nimenibo-Uadia, R. Effect of aqueous extract of Canavalia ensiformis seeds on hyperlipidaemia and hyperketonaemia in alloxan-induced diabetic rats. Biokemistri 2003, 15, 7–15. [Google Scholar]
- Kim, O.K.; Nam, D.-E.; You, Y.; Jun, W.; Lee, J. Protective effect of Canavalia gladiata on gastric inflammation induced by alcohol treatment in rats. J. Korean Soc. Food Sci. Nutr. 2013, 42, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Lee, D.H.; Kim, H.J.; Lee, S.J.; Ban, J.O.; Cho, M.C.; Jeong, H.S.; Yang, Y.; Hong, J.T.; Yoon, D.Y. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J. Nutr. Biochem. 2012, 23, 1552–1558. [Google Scholar] [CrossRef]
- Korea Food and Drug Administration Home Page. Analytical Methods of Korean Food Standards Codex. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_02.jsp?idx=263 (accessed on 17 June 2021).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chayaratanasin, P.; Caobi, A.; Suparpprom, C.; Saenset, S.; Pasukamonset, P.; Suanpairintr, N.; Barbieri, M.A.; Adisakwattana, S. Clitoria ternatea flower petal extract inhibits adipogenesis and lipid accumulation in 3T3-L1 preadipocytes by downregulating adipogenic gene expression. Molecules 2019, 24, 1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.-H.; Jegal, J.; Kim, Y.N.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry extract and its active polyphenols suppress adipogenesis in 3T3-L1 adipocytes and modulates fat accumulation and insulin resistance in diet-induced obese mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasello, B.; Malfa, G.A.; La Mantia, A.; Miceli, N.; Sferrazzo, G.; Taviano, M.F.; Giacomo, C.D.; Renis, M.; Acquaviva, R. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Loo, G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review). J. Nutr. Biochem. 2003, 14, 64–73. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Acangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed. Res. Int. 2019, 8748253. [Google Scholar] [CrossRef] [Green Version]
- Abdali, D.; Samson, S.E.; Grover, A.K. How effective are antioxidant supplements in obesity and diabetes? Med. Princ. Pract. 2015, 24, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyeon, H.; Min, C.W.; Moon, K.; Cha, J.; Gupta, R.; Park, S.U.; Kim, S.T.; Kim, J.K. Metabolic profiling-based evaluation of the fermentative behavior of Aspergillus oryzae and Bacillus subtilis for soybean residues treated at different temperatures. Foods 2020, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Han, S.S.; Hur, S.J.; Lee, S.K. A comparison of antioxidative and anti-inflammatory activities of sword beans and soybeans fermented with Bacillus subtilis. Food Funct. 2015, 6, 2736–2748. [Google Scholar] [CrossRef]
- Kim, J.-P.; Yang, Y.-S.; Kim, J.-H.; Lee, H.-H.; Kim, E.-S.; Moon, Y.-W.; Kim, J.-Y.; Chung, J.-K. Chemical properties and DPPH radical scavenging ability of sword bean (Canavalia gladiata) extract. Korean J. Food Sci. Technol. 2012, 44, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.-L.; Lim, Y.-W.; Radhakrishnan, A.K.; Lim, P.-E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauchi-Sato, K.; Ozeki, S.; Houjou, T.; Taguchi, R.; Fujimoto, T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002, 277, 44507–44512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, T.; Kobori, S.; Sakai, M.; Anami, Y.; Matsumura, T.; Matsuda, H.; Kasho, M.; Shichiri, M. β-very low density lipoprotein induces triglyceride accumulation through receptor mediated endocytotic pathway in 3T3-L1 adipocytes. Atherosclerosis 1997, 135, 57–64. [Google Scholar] [CrossRef]
- Hwang, J.W.; Do, H.J.; Kim, O.Y.; Chung, J.H.; Lee, J.-Y.; Park, Y.S.; Hwang, K.Y.; Seong, S.-I.; Shin, M.-J. Fermented soy bean extract suppresses differentiation of 3T3-L1 preadipocytes and facilitates its glucose utilization. J. Funct. Foods 2015, 15, 516–524. [Google Scholar] [CrossRef]
- So, K.-H.; Suzuki, Y.; Yonekura, S.; Suzuki, Y.; Lee, C.H.; Kim, S.W.; Katoh, K.; Roh, S.-G. Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes. Nutr. Res. Pract. 2015, 9, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, B.G.; Ong, J.M.; Kern, P.A. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid Res. 1994, 35, 1535–1541. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeong, J.E.; Moon, S.H.; Park, K.Y. Antiobesity effect of the Bacillus subtilis KC-3 fermented soymilk in 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1126–1131. [Google Scholar]
- Hauser, S.; Adelmant, G.; Sarraf, P.; Wright, H.M.; Mueller, E.; Spiegelman, B.M. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J. Biol. Chem. 2000, 275, 18527–18533. [Google Scholar] [CrossRef] [Green Version]
- Hertzel, A.V.; Bernlohr, D.A. The mammalian fatty acid-binding protein multigene family: Molecular and genetic insights into function. Trends Endocrinol. Metab. 2000, 11, 175–180. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Park, H.J.; Kang, S.N.; Jang, S.-H.; Lee, S.-J.; Ko, Y.-G.; Kim, G.-S.; Cho, J.-H. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS ONE 2013, 8, e69925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, C.-R.; Park, Y.-K.; Cha, Y.-S. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food Agric. 2014, 94, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Winder, W.W.; Hardie, D.G. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am. J. Physiol. 1999, 277, E1–E10. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef] [Green Version]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Liu, Y.; Zhang, F.; Zhao, Z.; Yu, Y.; et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.-Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.K.; Lopez, J.M.; Sanchez, H.B.; Osborne, T.F. Sterol regulation of fatty acid synthase promoter coordinate feedback regulation of two major lipid pathways. J. Biol. Chem. 1995, 270, 25578–25583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winder, W.W.; Wilson, H.A.; Hardie, D.G.; Rasmussen, B.B.; Hutber, C.A.; Call, G.B.; Clayton, R.D.; Conley, L.M.; Yoon, S.; Zhou, B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J. Appl. Physiol. 1997, 82, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Hwang, S.Y.; Han, J.S. Bamboo (Phyllostachys bambusoides) leaf extracts inhibit adipogenesis by regulating adipogenic transcription factors and enzymes in 3T3-L1 adipocytes. Food Sci. Biotech. 2017, 26, 1037–1044. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar] [PubMed]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Granados, O.; Torre-Villalvazo, I.; Serna-Saldivar, S.O.; Torres, N.; Palacios-González, B.; Tovar, A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014, 112, 886–899. [Google Scholar] [CrossRef] [Green Version]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S.; et al. Isoflavones derived from soy beans prevent MuRF1-mediated muscle atrophy in C2C12 myotubes through SIRT1 activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Kang, I.; Choi, S.; Ha, T.J.; Choi, M.; Wi, H.R.; Lee, B.W.; Lee, M. Effects of mung bean (Vigna radiata L.) ethanol extracts decrease proinflammatory cytokine-induced lipogenesis in the KK-Ay diabese mouse model. J. Med. Food 2015, 18, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.H.; Kang, N.H.; Mukherjee, S.; Yun, J.W. Theobromine, a methylxanthine in cocoa bean, stimulates thermogenesis by inducing white fat browning and activating brown adipocytes. Biotechnol. Bioprocess Eng. 2018, 23, 617–626. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Schoonjans, K.; Staels, B.; Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996, 37, 907–925. [Google Scholar] [CrossRef]
- Soh, J.-R.; Shin, D.-H.; Kwon, D.Y.; Cha, Y.-S. Effect of Cheonggukjang supplementation upon hepatic acyl-CoA synthase, carnitine palmitoyltransferase I, acyl-CoA oxidase and uncoupling protein 2 mRNA levels in C57BL/6J mice fed with high fat diet. Genes Nutr. 2008, 2, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.W.; Kim, Y.O.; Andrade, J.E.; Burgess, J.R.; Kim, Y.-C. Dietary naringenin increases hepatic peroxisome proliferators–activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. Eur. J. Nutr. 2011, 50, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.-N.; Park, S.-J.; Choue, R.; Lee, J. Standardized ethanol extract of Curcuma longa L. fermented by Aspergillus oryzae promotes lipolysis via activation of cAMP-dependent PKA in 3T3-L1 adipocytes. J. Food Biochem. 2013, 37, 595–603. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Ducharme, N.A.; Bickel, P.E. Minireview: Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, S.-T.; Yang, X.; You, P.-P.; Zhang, W. Myricetin suppresses differentiation of 3T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr. Res. 2015, 35, 317–327. [Google Scholar] [CrossRef]




| Target | Primer (5′→3′) | |
|---|---|---|
| aP2 | forward | AAGGTGAAGAGCATCATAACCCT |
| reverse | TCACGCCTTTCATAACACATTCC | |
| Adiponectin | forward | GCCTGTCCCCATGAGTAC |
| reverse | TCTTCGGCATGACTGGGC | |
| Ppara | forward | ACGATGCTGTCCTCCTTGATG |
| reverse | GCGTCTGACTCGGTCTTCTTG | |
| Acox1 | forward | GCACCTTCGAGGGGGAGAACA |
| reverse | GCGCGAACAAGGTCGACAGAA | |
| Lcad | forward | TCCGCCCGATGTTCTCATTC |
| reverse | AGGGCCTGTGCAATTTGAGT | |
| β-actin | forward | ACCCCAGCCATGTACGTAGC |
| reverse | GTGTGGGTGACCCCGTCTC | |
| Target | Secondary Host | Size (kDa) | Dilution | Company | Catalog No. |
|---|---|---|---|---|---|
| C/EBPα | Rabbit | 42 | 1:1000 | Cell signaling Technology | #2295 |
| PPARγ | Rabbit | 53, 57 | 1:1000 | Cell signaling Technology | #2443 |
| p-AMPK | Rabbit | 62 | 1:1000 | Cell signaling Technology | #2531 |
| AMPK | Rabbit | 62 | 1:1000 | Cell signaling Technology | #2532 |
| p-ACC | Rabbit | 280 | 1:1000 | Cell signaling Technology | #3661 |
| ACC | Rabbit | 280 | 1:1000 | Cell signaling Technology | #3676 |
| FAS | Rabbit | 273 | 1:1000 | Cell signaling Technology | #3180 |
| HSL | Rabbit | 81, 83 | 1:1000 | Cell signaling Technology | #4107 |
| p-HSL | Rabbit | 81, 83 | 1:1000 | Cell signaling Technology | #4139 |
| ATGL | Rabbit | 54 | 1:1000 | Cell signaling Technology | #2138 |
| Perilipin A | Rabbit | 62 | 1:1000 | Cell signaling Technology | #9349 |
| β-actin | Mouse | 45 | 1:1000 | Cell signaling | #3700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kim, D.-S.; Lee, M.-C.; Park, S.; Lee, J.-W.; Om, A.-S. Effects of Bacillus Subtilis-Fermented White Sword Bean Extract on Adipogenesis and Lipolysis of 3T3-L1 Adipocytes. Foods 2021, 10, 1423. https://doi.org/10.3390/foods10061423
Choi Y, Kim D-S, Lee M-C, Park S, Lee J-W, Om A-S. Effects of Bacillus Subtilis-Fermented White Sword Bean Extract on Adipogenesis and Lipolysis of 3T3-L1 Adipocytes. Foods. 2021; 10(6):1423. https://doi.org/10.3390/foods10061423
Chicago/Turabian StyleChoi, Yujeong, Da-Som Kim, Min-Chul Lee, Seulgi Park, Joo-Won Lee, and Ae-Son Om. 2021. "Effects of Bacillus Subtilis-Fermented White Sword Bean Extract on Adipogenesis and Lipolysis of 3T3-L1 Adipocytes" Foods 10, no. 6: 1423. https://doi.org/10.3390/foods10061423
APA StyleChoi, Y., Kim, D. -S., Lee, M. -C., Park, S., Lee, J. -W., & Om, A. -S. (2021). Effects of Bacillus Subtilis-Fermented White Sword Bean Extract on Adipogenesis and Lipolysis of 3T3-L1 Adipocytes. Foods, 10(6), 1423. https://doi.org/10.3390/foods10061423