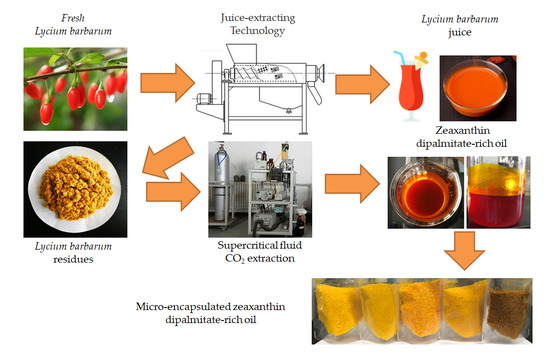
Supercritical Fluid CO2 Extraction and Microcapsule Preparation of Lycium barbarum Residue Oil Rich in Zeaxanthin Dipalmitate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Zeaxanthin Dipalmitate-Rich Oil
2.2.1. Preparation of Extraction Process
2.2.2. SFE-CO2 Extraction and Protocol Optimization
2.2.3. Quantification of Zeaxanthin Dipalmitate and Total Carotenoids
2.2.4. Analysis of Fatty Acids
2.3. Preparation and Analysis of Microcapsule
2.3.1. Emulsion Preparation
2.3.2. Spray-Drying Procedure
2.3.3. Microencapsulation Efficiency
2.3.4. Moisture Content
2.3.5. Wettability and Solubility
2.3.6. Scanning Electron Microscopy (SEM) Analysis
2.3.7. Accelerated Storage Test
2.4. Statistical Analysis
3. Results and Discussion
3.1. SFE-CO2 Extraction
3.1.1. SFE-CO2 Extraction Condition Optimizations
3.1.2. Analysis of Fatty-Acid Composition and Zeaxanthin Dipalmitate
3.2. Microcapsule Preparation
3.2.1. Powder Characteristics
3.2.2. Morphology
3.2.3. Storage Stability of Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharmaceut. Biomed. 2008, 47, 812–818. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Chung, W.Y.; Szeto, Y.T.; Benzie, I.F. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br. J. Nutr. 2005, 93, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, J.M.; Kim, J.; De Monasterio, F.; Thompson, D.J.; Bone, R.A.; Landrum, J.T.; De Moura, F.F.; Khachik, F.; Chen, H.; Schleicher, R.L. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5227–5233. [Google Scholar] [CrossRef] [PubMed]
- Trieschmann, M.; Beatty, S.; Nolan, J.M.; Hense, H.W.; Heimes, B.; Austermann, U.; Fobkerd, M.; Pauleikhoffa, D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin. Exp. Eye Res. 2007, 84, 718–728. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, C.; Li, Y.W.; Leung, K.S.; Jiang, Z.H.; Zhao, Z.Z. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Food Hum. Nutr. 2005, 60, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Shi, J.Y.; Suo, Y.R.; Sun, Z.W.; Xia, L.; Zheng, J.; You, J.M.; Liu, Y.J. Supercritical CO2 cell breaking extraction of Lycium barbarum seed oil and determination of its chemical composition by HPLC/APCI/MS and antioxidant activity. LWT Food Sci. Technol. 2011, 44, 1172–1178. [Google Scholar]
- Blasi, F.; Montesano, D.; Simonetti, M.S.; Cossignani, L. A simple and rapid extraction method to evaluate the fatty acid composition and nutritional value of goji berry lipid. Food Anal. Methods 2017, 10, 970–979. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, X.L.; Liu, Y.N.; Zhang, F.Q. Utilization of Microcapsule Technology in Foods. J. Nanosci. Nanotechnol. 2015, 15, 9330–9340. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Sandi, D. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar] [CrossRef]
- Brunner, G. Gas extraction: An introduction to fundamentals of supercritical fluids and the application to separation processes. In Topic in Physical Chemistry; Baumgurtel, H., Franck, E.U., Eds.; Springer: New York, NY, USA, 1994. [Google Scholar]
- Augustin, M.A.; Sanguansri, L.; Bode, O. Maillard reaction products as encapsulants for fish oil powders. J. Food Sci. 2006, 71, 25–32. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Cenkier, J.; S’wirydow, E. Selected properties of microencapsulated oil powders with commercial preparations of maize OSA starch and trehalose. J. Food Eng. 2015, 152, 72–84. [Google Scholar] [CrossRef]
- Juchen, P.T.; Arauj, M.N.; Hamerski, F.; Corazza, M.L.; Voll, F.A.P. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crop Prod. 2019, 139, 111506. [Google Scholar] [CrossRef]
- Karioti, A.; Bergonzi, M.C.; Vincieri, F.F.; Bilia, A.R. Validated method for the analysis of goji berry, a rich source of zeaxanthin dipalmitate. J. Agric. Food Chem. 2014, 62, 12529–12535. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Montesano, D.; De Angelis, M.; Maurizi, A.; Ventura, F.; Cossignani, L.; Simonetti, M.S.; Damiani, P. Results of stereospecific analysis of triacylglycerol fraction from donkey, cow, ewe, goat and buffalo milk. J. Food Comp. Anal. 2008, 21, 1–7. [Google Scholar] [CrossRef]
- Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food Anal. Methods 2018, 11, 1180–1188. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Dobarganes, C.; Márquez-Ruiz, G. Heterogeneous aspects of lipid oxidation in dried microencapsulated oils. J. Agric. Food Chem. 2006, 54, 1722–1729. [Google Scholar] [CrossRef]
- Pont, E.G. A de-emulsification technique for use in the peroxide test on the fat of milk, cream, concentrated and dried milks. Aust. J. Dairy Technol. 1955, 10, 72–75. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Fuchs, M.; Turchiuli, C.; Bohin, M.; Cuvelier, M.E.; Ordonnaud, C.; Peyrat-Maillard, M.N.; Dumoulin, E. Encapsulation of oil in powder using spraydrying and fluidised bed agglomeration. J. Food Eng. 2006, 75, 27–35. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of thecarriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silvaa, V.M.; Tonona, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Khazaei, K.M.; Jafari, S.; Ghorbani, M.; & Kakhki, A.H. Application of maltodextrin and gum arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr. Polym. 2014, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Mayamol, P.N.; Thomas, S.; Sukumar, D.; Sundaresan, A.; Arumughan, C. An ecofriendly approach to process rice bran for high quality rice bran oil using supercritical carbon dioxide for nutraceutical applications. Bioresour. Technol. 2008, 99, 2905–2912. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Wahyudiono, F.R.; Kanda, H.; Quitain, A.T.; Sasaki, M.; Goto, M. Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 2014, 125, 319–325. [Google Scholar] [CrossRef]
- Trebatická, J.; Dukát, A.; Ďuračkovác, Z.; Muchovác, J. Cardiovascular diseases, depression disorders and potential effects of omega-3 fatty acids. Physiol. Res. 2017, 66, 363–382. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; Fernandes, R.V.B.; Viana, A.D.; Costa, J.M.G.; Marques, G.R. Evaluation of spray drying conditions on properties of microencapsulated oregano essential oil. Int J Food Sci Tech 2012, 47, 2289–2296. [Google Scholar] [CrossRef]
- Cuq, B.; Rondet, E.; Abecassis, J. Food powders engineering, between knowhow and science: Constraints, stakes and opportunities. Powder Technol. 2011, 208, 244–251. [Google Scholar] [CrossRef]
- Jayasundera, M.; Adhikari, B.; Howes, T.; Aldred, P. Surface protein coverage and its implications on spray-drying of model sugar-rich foods: Solubility, powder production and characterization. Food Chem. 2011, 128, 1003–1016. [Google Scholar] [CrossRef]
- Jafariac, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 26816–26835. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Turatti, I.C.C.; Lopes, N.P.; Ferreira, J.C.; Candido, R.C.; Oliveira, W.P. Volatile retention and antifungal properties of spray-dried micro particles of Lippia sidoides essential oil. Dry. Technol. 2008, 26, 1534–1542. [Google Scholar] [CrossRef]
- Bule, M.V.; Singhal, R.S.; Kennedy, J.F. Microencapsulation of ubiquinone-10 in carbo-hydrate matrices for improve stability. Carbohydr. Polym. 2010, 82, 1290–1296. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Microencapsulation by spray drying: Influence of emulsion size on the retention of volatile compounds. J. Food Sci. 2003, 68, 2256–2262. [Google Scholar] [CrossRef]
- Wang, R.X.; Tian, Z.G.; Chen, L.Y. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]




| No. | Wall Material (g 100 g−1 of Solution) | Core Material (g 100 g−1 of Solution) | Solid Content (%) | ||||
|---|---|---|---|---|---|---|---|
| OSA Starch (OSA) | Gum Arabic (GA) | Maltodextrin (MD) | Soy Isolate Protein (SPI) | L. barbarum Polysaccharide (LPS) | L. barbarum Seed Oil | ||
| 1 | 24.0 | 6.0 | 30.0 | ||||
| 2 | 12.0 | 12.0 | 6.0 | 30.0 | |||
| 3 | 12.0 | 12.0 | 6.0 | 30.0 | |||
| 4 | 13.5 | 7.5 | 3.0 | 6.0 | 30.0 | ||
| 5 | 13.5 | 7.5 | 3.0 | 6.0 | 30.0 | ||
| Extraction Conditions | Optimum | Zeaxanthin Dipalmitate Yield (‰) | Oil Yield (%) |
|---|---|---|---|
| Extraction pressure | 250 bar | 0.26 ± 0.05 a | 17.0 ± 0.67 a |
| Extraction temperature | 60 °C | ||
| Dynamic extraction time | 2.0 h | ||
| CO2 flow | 30 g/min | ||
| Cosolvent | 2% ethanol | 0.85 ± 0.11 b | 15.2 ± 0.42 b |
| FAs | mg/mg (oil) a | Relative Content (%) b |
|---|---|---|
| Linoleic acid (C18:2) | 430.26 ± 8.12 | 65.18 ± 0.89 |
| Oleic acid (C18:1) | 146.02 ± 5.20 | 22.12 ± 0.75 |
| γ-Linolenic acid (C18:3) | 30.04 ± 1.81 | 4.55 ± 0.30 |
| UFAs | 606.32 ± 6.61 | 91.85 ± 0.27 |
| Palmitic acid (C16:0) | 21.52 ± 1.15 | 3.26 ± 0.20 |
| Stearic acid (C18:0) | 32.28 ± 0.33 | 4.89 ± 0.07 |
| SFAs | 53.80 ± 1.41 | 8.15 ± 0.27 |
| TFAs | 660.12 ± 5.47 | 100 ± 0.00 |
| No. | Wall Material | Variables | |||
|---|---|---|---|---|---|
| Moisture (%) | Wettability (s) | Solubility (%) | MEE (%) | ||
| 1 | OSA-starch | 2.02 ± 0.17 a | 256 ± 5 a | 64.96 ± 0.35 a | 68.85 ± 0.25 a |
| 2 | OSA-starch:GA | 2.33 ± 0.22 b | 301 ± 8 b | 62.65 ± 0.37 b | 82.22 ± 0.18 b |
| 3 | OSA-starch:SPI | 2.79 ± 0.09 c | 485 ± 12 c | 55.12 ± 0.45 c | 76.92 ± 0.05 c |
| 4 | OSA-starch:GA:MD | 1.98 ± 0.05 a | 298 ± 11 b | 66.22 ± 0.24 d | 92.83 ± 0.13 d |
| 5 | OSA-starch:GA:LPS | 2.59 ± 0.16 c | 322 ± 10 e | 52.13 ± 0.16 e | 83.92 ± 0.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men, Y.; Fu, S.; Xu, C.; Zhu, Y.; Sun, Y. Supercritical Fluid CO2 Extraction and Microcapsule Preparation of Lycium barbarum Residue Oil Rich in Zeaxanthin Dipalmitate. Foods 2021, 10, 1468. https://doi.org/10.3390/foods10071468
Men Y, Fu S, Xu C, Zhu Y, Sun Y. Supercritical Fluid CO2 Extraction and Microcapsule Preparation of Lycium barbarum Residue Oil Rich in Zeaxanthin Dipalmitate. Foods. 2021; 10(7):1468. https://doi.org/10.3390/foods10071468
Chicago/Turabian StyleMen, Yan, Shaoping Fu, Chao Xu, Yueming Zhu, and Yuanxia Sun. 2021. "Supercritical Fluid CO2 Extraction and Microcapsule Preparation of Lycium barbarum Residue Oil Rich in Zeaxanthin Dipalmitate" Foods 10, no. 7: 1468. https://doi.org/10.3390/foods10071468
APA StyleMen, Y., Fu, S., Xu, C., Zhu, Y., & Sun, Y. (2021). Supercritical Fluid CO2 Extraction and Microcapsule Preparation of Lycium barbarum Residue Oil Rich in Zeaxanthin Dipalmitate. Foods, 10(7), 1468. https://doi.org/10.3390/foods10071468