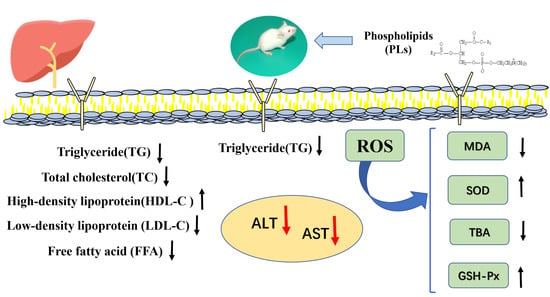
Comparison of Egg Yolk and Soybean Phospholipids on Hepatic Fatty Acid Profile and Liver Protection in Rats Fed a High-Fructose Diet
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Animals and Diets
2.3. Body Weight, Food Intake, and Liver, Abdominal Fat, and Brain Weight
2.4. Histopathological Observation of the Liver
2.5. Analysis of Lipid Parameters
2.6. Determination of MDA, TBA, CRP Concentration, and SOD Activity
2.7. Phospholipids and Related Metabolites
2.8. Analysis of Fatty Acids of PLs and Liver
2.9. Statistical Analysis
3. Results
3.1. Fatty Acid Composition
3.2. Effects of Dietary PLs on Body and Tissue Weights
3.3. Hepatic Histopathological Observations
3.4. Effects of Dietary PLs on Lipid Metabolism
3.5. Oxidation and the Anti-Inflammatory Factor
3.6. Change in the Hepatic Fatty Acid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| ANOVA | Analysis of variance |
| AST | Aspartate transaminase |
| EPLs | Egg yolk phospholipids |
| FAME | Fatty acid methyl esters |
| FFA | Free fatty acid |
| FT-IR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| H&E | Hematoxylin and eosin |
| HFD | High-fructose diet |
| PCA | Principal component analysis |
| SEM | Scanning electron microscope |
| SD | Sprague Dawley |
| SPLs | Soya bean phospholipids |
| TC | Total cholesterol |
| TG | Triglyceride |
| VIP | Variable importance in projection |
References
- Passero, K.; He, X.; Zhou, J.; Mueller-Myhsok, B.; Kleber, M.; Maerz, W.; Hall, M. Phenome-wide association studies on cardiovascular health and fatty acids considering phenotype quality control practices for epidemiological data. Biocomputing 2020, 25, 659–670. [Google Scholar]
- Francisco, J.A.; Jeroen, H.B.J. CrossTalk opposing view: CNNM proteins are not Na+/Mg2+ exchangers but Mg2+ transport regulators playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. 2018, 596, 747–750. [Google Scholar]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.; Schoelles, K. Bariatric Surgery: A Systematic Review and Meta-analysis. ACC Curr. J. Rev. 2004, 14, 13. [Google Scholar] [CrossRef]
- Eaton, C.B. Hyperlipidemia. Prim. Care Clin. Off. Pract. 2005, 32, 1027–1055. [Google Scholar] [CrossRef]
- Daas, I.D.; Wemer, J.; Farha, K.A.; Tamminga, W.; de Boer, T.; Spanjersberg, R.; Struys, M.M.R.F.; Absalom, A.R. Serial CSF sampling over a period of 30 h via an indwelling spinal catheter in healthy volunteers: Headache, back pain, tolerability and measured acetylcholine profile. Eur. J. Clin. Pharmacol. 2013, 69, 1083–1090. [Google Scholar] [CrossRef]
- Canty, D.J.; Zeisel, S.H. Lecithin and Choline in Human Health and Disease. Nutr. Rev. 1994, 10, 327–339. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Liu, B.; Kai, H.; Huang, K. Enoyl coenzyme A hydratase 1 protects against high-fat-diet-induced hepatic steatosis and insulin resistance. Biochem. Biophys. Res. Commun. 2018, 499, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Han, L.D.; Xia, J.F.; Liang, Q.L.; Wang, Y.; Wang, Y.M.; Hu, P.; Luo, G.A. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography-mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta 2011, 689, 85–91. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Oh, K.W. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Han, X.; Huang, D.; Lu, Y.; Yang, X. Enhancing the hepatic protective effect of genistein by oral administration with stachyose in mice with chronic high fructose diet consumption. Food Funct. 2016, 7, 2420–2430. [Google Scholar] [CrossRef] [PubMed]
- Martins-Oliveira, A.; Guimaraes, D.A.; Ceron, C.S.; Rizzi, E.; Tanus-Santos, J.E. Direct renin inhibition is not enough to prevent reactive oxygen species generation and vascular dysfunction in renovascular hypertension. Eur. J. Pharmacol. 2018, 821, 97–104. [Google Scholar] [CrossRef]
- Marchbanks, R.M. The subcellular origin of the agetylcholine released at synapses. Int. J. Biochem. 1975, 6, 303–312. [Google Scholar] [CrossRef]
- Folch, J.M.; Lee, S.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J.Biol.Chem. 1975, 226, 497–509. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chang, W.T.; Li, S.; Wu, J.C.; Badmeav, V.; Ho, C.T.; Pan, M.H. Citrus peel extracts attenuated obesity and modulated gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2018, 9, 3363–3373. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, L.; Jiang, G.; Liu, Q. Mechanism of the Protective Effect of Sesamin on Sepsis-Induced Acute Lung Injury. Curr. Top. Nutraceutical Res. 2021, 2, 211–216. [Google Scholar]
- El-Ouady, F.; Ayoub, A.; Mohamed, E. Mohamed Eddouks. Antihyperglycemic and Antidyslipidemic Activities of the Aqueous Salvia hispanica Extract in Diabetic Rat. Cardiovasc. Hematol. Agents Med. Chem. 2021. [Google Scholar] [CrossRef]
- David, B.; David, N.; Joe, S.; Harvey, G.; Dean, S.E.; Luke, T.L.; Paul, M.; Kristen, M.; Ryan, P.; Hassan, R.; et al. Development of a patient decision aid for the surgical management of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU Int. 2021, 1, 131–135. [Google Scholar]
- Young, S. Prostate artery embolization for benign prostatic hyperplasia: The hunt for the ideal patient population. Diagn. Interv. Imaging 2021, 102, 119–120. [Google Scholar] [CrossRef]
- Vandriel, M.; Morledge, M.; Ulep, R.; Shaffer, J.; Davies, P.; Deichmann, R. Cochrane corner: Interventions to improve adherence to lipid-lowering medication. Heart 2018, 5, 367–369. [Google Scholar] [CrossRef]
- Negrini, S.; Arienti, C.; Kiekens, C. Cochrane Rehabilitation and the future of systematic reviews in developmental rehabilitation. Dev. Med. Child Neurol. 2019, 11, 1241. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.; Poitout, V. β-Cell Glucose Toxicity, Lipotoxicity, and Chronic Oxidative Stress in Type 2 Diabetes. Diabetes 2004, 53, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, B.; Streich, S.; Schonthier, U.M.; Richter, W.O.; Duhm, J. Changes of membrane phospholipid composition of human erythrocytes in hyperlipidemias. I. Increased phosphatidylcholine and reduced sphingomyelin in patients with elevated levels of triacylglycerol-rich lipoproteins. Biochim. Biophys. Acta 1992, 1165, 32–37. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, L.; Wen, M.; Che, H.; Huang, J.; Zhang, T.; Xue, C.; Mao, X.; Wang, Y. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Kesh, S.B.; Sikder, K.; Manna, K.; Das, D.K.; Dey, S. Promising role of ferulic acid, atorvastatin and their combination in ameliorating high fat diet-induced stress in mice. Life Sci. 2013, 92, 938–949. [Google Scholar] [CrossRef]
- Amanda, L.B.; Kelsey, C.; Daniela, S.A.; Anthony, D.G.; Renliang, Z.; Chase, K.; Phillip, O.A.; Michael, T.; Robert, N.H. Dietary Choline Supplementation Attenuates High-Fat-Diet-Induced Hepatocellular Carcinoma in Mice. J. Nutr. 2020, 150, 775–783. [Google Scholar]
- Erwann, D.; André, D.; Claire, B.; Jérome, L.; Bruno, B.; Maria, C.F.E.; Eric, A.D.; Francoise, M.S.; Gilles, K.; Pierre, V. Eleostearic phospholipids as probes to evaluate antioxidants efficiency against liposomes oxidation. Chem. Phys. Lipids 2017, 209, 19–28. [Google Scholar]
- Che, H.X.; Fu, X.Y.; Zhang, L.Y.; Gao, X.; Wen, M.; Du, L.; Xue, C.H.; Xu, J.; Wang, Y.M. Neuroprotective Effects of n-3 Polyunsaturated Fatty Acid-Enriched Phosphatidylserine Against Oxidative Damage in PC12 Cells. Cell. Mol. Neurobiol. 2018, 38, 657–668. [Google Scholar] [CrossRef]
- Greig, F.H.; Kennedy, S.; Spickett, C.M. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic. Biol. Med. 2012, 52, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Ronan, L.; Aaron, W.; Fiona, C.; Laura, F.; Martina, D.; Alexandros, T.; Paul, D.C.; Ioannis, Z. Caprine milk fermentation enhances the antithrombotic properties of cheese polar lipids. J. Funct. Foods 2019, 61, 103507. [Google Scholar]
- Mariano, C.; Michele, A.; Andrea, A.; Vanessa, C.; Margherita, A.; Elisabetta, B.; Giovambattista, D.; Annamaria, C. Neuroprotective potential of choline alfoscerate against β-amyloid injury: Involvement of neurotrophic signals. Cell Biol. Int. 2020, 44, 1734–1744. [Google Scholar]



| Components | Control Group | High-Fructose Group | EP | SP |
|---|---|---|---|---|
| Background diet | 100 | 0 | 0 | 0 |
| Sucrose | 0 | 45.4 | 45.4 | 45.4 |
| Casein | 0 | 20 | 20 | 20 |
| β-corn starch | 0 | 15 | 15 | 15 |
| Cellulose | 0 | 5 | 5 | 5 |
| Mineral mixture (AIN-76) | 0 | 3.5 | 3.5 | 3.5 |
| Vitamin blend | 0 | 1 | 1 | 1 |
| Corn oil | 0 | 10 | 8 | 8 |
| Soy bean phospholipid | 0 | 0 | 0 | 2 |
| Egg yolk phospholipid (PL-100M) | 0 | 0 | 2.04 | 0 |
| Cholesterol | 0 | 0.1 | 0.06 | 0.1 |
| SUM | 100 | 100 | 100 | 100 |
| Control | High-Fructose Group | EP | SP | ||
|---|---|---|---|---|---|
| Growth parameters | |||||
| Body weight, initial (g) | 101.03 ± 8.31 | 99.57 ± 5.91 | 102.59 ± 7.94 | 99.79 ± 5.72 | |
| Body weight, final (g) | 257.70 ± 7.98 c | 281.65 ± 5.89 a | 274.56 ± 4.27 b*** | 290.46 ± 6.51 a | |
| Food intake (g/d) | 16.52 ± 0.11 | 16.37 ± 0.12 | 16.44 ± 0.32 | 16.45 ± 0.25 | |
| Food efficiency (%) | 34.81 ± 0.88 c | 38.73 ± 1.17 ab | 37.05 ± 1.42 b** | 39.97 ± 2.25 a | |
| Liver weight (g) | 8.32 ± 0.81 b | 10.75 ± 1.39 a | 9.08 ± 0.54 b | 8.88 ± 0.53 b | |
| Abdominal fat weight (g) | 0.90 ± 0.40 c | 3.66 ± 0.78 a | 2.37 ± 0.58 b* | 3.55 ± 1.12 a | |
| Control | High-Fructose Group | EP | SP | ||
|---|---|---|---|---|---|
| Serum parameters | |||||
| TG (mg/dL) | 27.40 ± 5.93 b | 39.07 ± 8.43 a | 24.73 ± 7.34 b | 30.07 ± 7.45 b | |
| TC (mmol/L) | 14.74 ± 4.80 | 15.85 ± 2.64 | 16.40 ± 3.15 | 17.08 ± 6.53 | |
| HDL-C (μmol/dL) | 32.38 ± 5.09 ab | 19.50 ± 4.28 c | 35.70 ± 11.62 a* | 25.37 ± 2.94 bc | |
| LDL-C (μmol/dL) | 8.32 ± 0.79 b | 11.20 ± 1.08 a | 8.79 ± 0.95 a | 8.43 ± 1.70 a | |
| FFA (mmol/dL) | 19.48 ± 9.72 c | 65.03 ± 10.87 a | 30.74 ± 13.77 bc | 39.96 ± 14.21 b | |
| PLs (mg/dL) | 110.93 ± 48.36 b | 128.1 ± 75.27 b | 189.77 ± 44.56 a | 140.6 ± 42.15 ab | |
| Cho (nmol/mL) | 13.59 ± 3.03 a | 6.44 ± 0.60 c | 10.53 ± 1.71 ab* | 9.42 ± 1.31 bc | |
| ACH (nmol/mL) | 2.52 ± 0.65 ab | 0.92 ± 0.46 c | 3.49 ± 0.58 a** | 1.81 ± 0.61 bc | |
| Hepatic parameters | |||||
| TG (mg/g) | 3.81 ± 2.16 c | 17.74 ± 3.01 a | 7.59 ± 4.89 bc | 9.65 ± 5.28 b | |
| TC (μmol/g) | 8.80 ± 3.37 c | 38.22 ± 3.95 b | 44.06 ± 4.53 a | 15.03 ± 6.58 ab | |
| Cho (nmol/g) | 24.9 ± 6.67 a | 13.24 ± 1.13 b | 18.86 ± 3.81 ab | 15.18 ± 7.16 b | |
| ACH (nmol/g) | 10.9 ± 3.76 a | 4.89 ± 1.17 b | 8.52 ± 0.13 ab | 6.64 ± 0.56 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Matsuoka, R.; Xi, Y.; Wang, X. Comparison of Egg Yolk and Soybean Phospholipids on Hepatic Fatty Acid Profile and Liver Protection in Rats Fed a High-Fructose Diet. Foods 2021, 10, 1569. https://doi.org/10.3390/foods10071569
Yin M, Matsuoka R, Xi Y, Wang X. Comparison of Egg Yolk and Soybean Phospholipids on Hepatic Fatty Acid Profile and Liver Protection in Rats Fed a High-Fructose Diet. Foods. 2021; 10(7):1569. https://doi.org/10.3390/foods10071569
Chicago/Turabian StyleYin, Mingyu, Ryosuke Matsuoka, Yinci Xi, and Xichang Wang. 2021. "Comparison of Egg Yolk and Soybean Phospholipids on Hepatic Fatty Acid Profile and Liver Protection in Rats Fed a High-Fructose Diet" Foods 10, no. 7: 1569. https://doi.org/10.3390/foods10071569
APA StyleYin, M., Matsuoka, R., Xi, Y., & Wang, X. (2021). Comparison of Egg Yolk and Soybean Phospholipids on Hepatic Fatty Acid Profile and Liver Protection in Rats Fed a High-Fructose Diet. Foods, 10(7), 1569. https://doi.org/10.3390/foods10071569