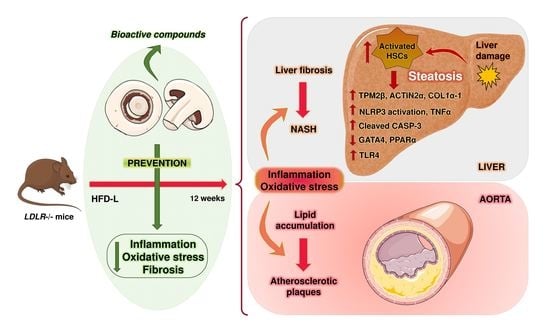
White Button Mushroom Extracts Modulate Hepatic Fibrosis Progression, Inflammation, and Oxidative Stress In Vitro and in LDLR-/- Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Chemical Characterization of Natural Aqueous Mushroom Extract
2.2. Cell Cultures
2.3. Animal Diets and Experimental Design
2.4. Standard Biological Parameters
2.5. Gene Expression Analysis
2.6. Western Blot Analysis
2.7. Liver Histology
2.8. Injury Analysis of the Aorta
2.9. Statistical Analysis
3. Results
3.1. Treatment of LX2 Cells with AB Extract Reduced the Levels of Fibrotic and Oxidative Stress Markers and Increased the Levels of GATA4
3.2. AB Extract Inhibited the Increases in BW, Glycaemia, and Transaminase Levels in LDLR-/- Mice Fed an HFD Containing 60% Calories from Fats
3.3. Liver Fibrosis, Inflammation, and Apoptosis Were Decreased in LDLR-/- Mice Treated with AB Extract after 12 Weeks of HFD Feeding
3.4. AB Extract Reduced the Liver Expression of Genes Involved in Fibrosis, Oxidative Stress, and Inflammation in LDLR-/- Mice after 12 Weeks of HFD Feeding
3.5. LDLR-/- Mice in the HFD60+AB Group Did Not Show More Aortic Atherosclerotic Lesions despite Higher Fat and Caloric Contents in Their Diet
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Salic, K.; Verheij, J.; Liang, W.; Van Duyvenvoorde, W.; Menke, A.; Kooistra, T.; Kleemann, R.; Wielinga, P.Y. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis and hepatic fibrosis in LDLR−/−.Leiden mice. Int. J. Obes. 2016, 40, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongiovanni, P.; Lanti, C.; Riso, P.; Valenti, L. Nutritional therapy for nonalcoholic fatty liver disease. J. Nutr. Biochem. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Kiziltas, S. Toll-like receptors in pathophysiology of liver diseases. World J. Hepatol. 2016, 8, 1354–1369. [Google Scholar] [CrossRef]
- Mcgettrick, A.F.; O’Neill, L.A.J. NLRP3 and IL-1β in macrophages as critical regulators of metabolic diseases. Diabetes Obes. Metab. 2013, 15, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fesslerb, M.B.; Rudela, L.L.; Brown, M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Bone 2008, 23. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wu, X.; Ma, Y.; Shao, F.; Tan, Y.; Tan, T.; Gu, L.; Zhou, Y.; Sun, B.; Sun, Y.; et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Otogawa, K.; Ogawa, T.; Shiga, R.; Ikeda, K.; Kawada, N. Induction of tropomyosin during hepatic stellate cell activation and the progression of liver fibrosis. Hepatol. Int. 2009, 3, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, S.; Palčić, M.G.; Jukić, L.V.; Duvnjak, L.; Duvnjak, M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 18070–18091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Luetjohann, D.; Kerksiek, A.; Van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.C.; Chen, S.-C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.-H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 2011, 8, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Nagao, K.; Inoue, N.; Inafuku, M.; Shirouchi, B.; Morooka, T.; Nomura, S.; Nagamori, N.; Yanagita, T. Mukitake mushroom (Panellus serotinus) alleviates nonalcoholic fatty liver disease through the suppression of monocyte chemoattractant protein 1 production in db/db mice. J. Nutr. Biochem. 2010, 21, 418–423. [Google Scholar] [CrossRef]
- Delgado, I.; Carrasco, M.; Cano, E.; Carmona, R.; Garcia-Carbonero, R.; Marin-Gomez, L.M.; Soria, B.; Martin, F.; Cano, D.A.; Munoz-Chapuli, R.; et al. GATA4 loss in the septum transversum mesenchyme promotes liver fibrosis in mice. Hepatology 2014, 59, 2358–2370. [Google Scholar] [CrossRef]
- López-Tenorio, I.I.; Domínguez-López, A.; Miliar-García, Á.; Escalona-Cardoso, G.N.; Real-Sandoval, S.A.; Gómez-Alcalá, A.; Jaramillo-Flores, M.E. Modulation of the mRNA of the Nlrp3 inflammasome by Morin and PUFAs in an obesity model induced by a high-fat diet. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, L.; Jiang, K. Propofol attenuates inflammatory response and apoptosis to protect d-galactosamine/lipopolysaccharide induced acute liver injury via regulating TLR4/NF-κB/NLRP3 pathway. Int. Immunopharmacol. 2019, 77. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Pei, C.; Liang, J.; Ding, P.; Chen, S.; Hou, S.-Z. Monotropein alleviates secondary liver injury in chronic colitis by regulating TLR4/NF-κB signaling and NLRP3 inflammasome. Eur. J. Pharmacol. 2020, 883. [Google Scholar] [CrossRef]
- Izaola, O.; de Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflamación y obesidad (lipoinflamación). Nutr. Hosp. 2015, 31, 2352–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inafuku, M.; Nagao, K.; Nomura, S.; Shirouchi, B.; Inoue, N.; Nagamori, N.; Nakayama, H.; Toda, T.; Yanagita, T. Protective effects of fractional extracts from Panellus serotinus on non-alcoholic fatty liver disease in obese, diabetic db/db mice. Br. J. Nutr. 2012, 107, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Kim, H.G.; Son, C.G. Tissue-specific profiling of oxidative stress-associated transcriptome in a healthy mouse model. Int. J. Mol. Sci. 2018, 19, 3174. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wang, T.; Sun, B.; Liu, D.; Lin, Z.; Miao, Y.; Wang, C.; Geng, X.; Li, B. Xian-Ling-Gu-Bao induced inflammatory stress rat liver injury: Inflammatory and oxidative stress playing important roles. J. Ethnopharmacol. 2019, 239. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Xu, M.; Chen, L.; Liu, Q.; Zhou, Y.; Sun, Z.; Ye, H.; Su, N.; Ye, C.; Wang, A. Xiaochaihu Decoction reduces hepatic steatosis and improves D-GalN/LPS-induced liver injury in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol. 2019, 91, 293–305. [Google Scholar] [CrossRef]
- Barreyro, F.J.; Holod, S.; Finocchietto, P.V.; Camino, A.M.; Aquino, J.B.; Avagnina, A.; Carreras, M.C.; Poderoso, J.J.; Gores, G.J. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015, 35, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a Mediator of Non-Alcoholic Fatty Liver Disease and Associated Atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.; et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Vishnu, A.; Gurka, M.J.; DeBoer, M.D. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: The Atherosclerosis Risk in Communities Study. Atherosclerosis 2015, 243, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Sierra, A.L.; Alvarez-Amor, L.; Kleemann, R.; Martin, F.; Varela, L.M. Extra-Virgin Olive Oil with Natural Phenolic Content Exerts an Anti-Inflammatory Effect in Adipose Tissue and Attenuates the Severity of Atherosclerotic Lesions inLdlr−/−.Leiden Mice. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Johnston, S.L.; Souter, D.M.; Tolkamp, B.J.; Gordon, I.J.; Illius, A.W.; Kyriazakis, I.; Speakman, J.R. Intake compensates for resting metabolic rate variation in female C57BL/6J mice fed high-fat diets. Obesity 2007, 15, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Paulino, G.; Raybould, H.E. Activation of hindbrain neurons in response to gastrointestinal lipid is attenuated by high fat, high energy diets in mice prone to diet-induced obesity. Brain Res. 2009, 1248, 136–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhofer, A.; Wernly, B.; Leitner, L.; Sarabi, A.; Sommer, N.G.; Staffler, G.; Zeyda, M.; Stulnig, T.M. An accelerated mouse model for atherosclerosis and adipose tissue inflammation. Cardiovasc. Diabetol. 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, N.; Bannitz, R.; Silva, B.; Becari, D.; Poloni, C.; Gomes, P.; Foss, M.; Foss-Freitas, M. α-Linolenic acid prevents hepatic steatosis and improves glucose tolerance in mice fed a high-fat diet. Clinics 2018, 73. [Google Scholar] [CrossRef]
- Gunawardena, D.; Bennett, L.; Shanmugam, K.; King, K.; Williams, R.; Zabaras, D.; Head, R.; Ooi, L.; Gyengesi, E.; Münch, G. Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-γ activated murine macrophages. Food Chem. 2014, 148, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Su, L.; Wang, Q.; Li, Y. Protective effect of polysaccharide from Agaricus bisporus in Tibet area of China against tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2018, 118, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56. [Google Scholar] [CrossRef]
- Cremades, O.; Diaz-Herrero, M.M.; Carbonero-Aguilar, P.; Gutierrez-Gil, J.F.; Fontiveros, E.; Morgado, B.R.; Parrado, J.; Bautista, J. Preparation and characterisation of selenium-enriched mushroom aqueous enzymatic extracts (MAEE) obtained from the white button mushroom (Agaricus bisporus). Food Chem. 2012, 133, 1538–1543. [Google Scholar] [CrossRef]
- Van Koppen, A.; Verschuren, L.; Hoek, A.M.V.D.; Verheij, J.; Morrison, M.C.; Li, K.; Nagabukuro, H.; Costessi, A.; Caspers, M.P.; Broek, T.J.V.D.; et al. Uncovering a Predictive Molecular Signature for the Onset of NASH-Related Fibrosis in a Translational NASH Mouse Model. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Schoemaker, M.H.; Kleemann, R.; Morrison, M.C.; Verheij, J.; Salic, K.; Van Tol, E.A.F.; Kooistra, T.; Wielinga, P.Y. A casein hydrolysate based formulation attenuates obesity and associated non-alcoholic fatty liver disease and atherosclerosis in LDLr-/-.Leiden mice. PLoS ONE 2017, 12, e0180648. [Google Scholar] [CrossRef]
- Bieghs, V.; van Gorp, P.J.; Wouters, K.; Hendrikx, T.; Gijbels, M.J.; Van Bilsen, M.; Bakker, J.; Binder, C.J.; Luetjohann, D.; Staels, B.; et al. LDL Receptor Knock-Out Mice Are a Physiological Model Particularly Vulnerable to Study the Onset of Inflammation in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2012, 7, e30668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, A.; Kong, S.W.; Agarwal, P.; Gilliss, B.; Pu, W.T.; Black, B.L. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol. Cell. Biol. 2008, 28, 5420–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, P.; Liang, W.; Wielinga, P.Y.; Verschuren, L.; Toet, K.; Havekes, L.M.; Hoek, A.M.V.D.; Kleemann, R. Macrovesicular steatosis is associated with development of lobular inflammation and fibrosis in diet-induced non-alcoholic steatohepatitis (NASH). Inflamm. Cell Signal. 2015, 2. [Google Scholar] [CrossRef]
- Fontes, A.; Alemany-Pagès, M.; Oliveira, P.J.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Antioxidant versus pro-apoptotic effects of mushroom-enriched diets on mitochondria in liver disease. Int. J. Mol. Sci. 2019, 20, 3987. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Wang, J.; Xie, M.; Lu, Z.; Xu, H.; Shi, J.-S.; Xu, Z.-H. Screening and isolation for anti-hepatofibrotic components from medicinal mushrooms using TGF-(β1-induced live fibrosis in hepatic stellate cells. Int. J. Med. Mushrooms 2014, 16, 529–539. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.-M. Review of Pharmacological Effects ofAntrodia camphorataand Its Bioactive Compounds. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Chirumbolo, S. Hormesis, resveratrol and plant-derived polyphenols: Some comments. Hum. Exp. Toxicol. 2011, 30, 2027–2030. [Google Scholar] [CrossRef]
- Panthong, S.; Boonsathorn, N.; Chuchawanku, S. Antioxidant activity, anti-proliferative activity, and amino acid profiles of ethanolic extracts of edible mushrooms. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Kanaya, N.; Kubo, M.; Liu, Z.; Chu, P.; Wang, C.; Yuan, S.C.Y.-C. Protective Effects of White Button Mushroom (Agaricus bisporus) against Hepatic Steatosis in Ovariectomized Mice as a Model of Postmenopausal Women. PLoS ONE 2011, 6, e26654. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, N.; Yoshimoto, H.; Kurihara, S.; Hamaya, T.; Eguchi, F. Improvement of Diet-induced Obesity by Ingestion of Mushroom Chitosan Prepared from Flammulina velutipes. J. Oleo Sci. 2018, 67, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hritz, I.; Mandrekar, P.; Velayudham, A.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Kurt-Jones, E.; Szabo, G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatolology 2008, 48, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Tapia, N.C.; González-Rodríguez, L.; Jeong, M.S.; López-Ramírez, Y.; Barbero-Becerra, V.; Juárez-Hernández, E.; Romero-Flores, J.L.; Arrese, M.; Méndez-Sánchez, N.; Uribe, M. Current evidence on the use of probiotics in liver diseases. J. Funct. Foods 2015, 17, 137–151. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Seki, E.; Brenner, D.A. Toll-Like Receptor Signaling in the Liver. Gastroenterology 2006, 130, 1886–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einer, C.; Leitzinger, C.; Lichtmannegger, J.; Eberhagen, C.; Rieder, T.; Borchard, S.; Wimmer, R.; Denk, G.; Popper, B.; Neff, F.; et al. A High-Calorie Diet Aggravates Mitochondrial Dysfunction and Triggers Severe Liver Damage in Wilson Disease Rats. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 571–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taverne, Y.J.H.J.; Bogers, A.J.J.C.; Duncker, D.J.; Merkus, D. Reactive Oxygen Species and the Cardiovascular System. Oxidative Med. Cell. Longev. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.; Baker, R.; Zhu, L. Antioxidant Mechanisms in Nonalcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1301–1314. [Google Scholar] [CrossRef]
- Álvarez-Amor, L.; Sierra, A.L.; Cárdenas, A.; López-Bermudo, L.; López-Beas, J.; Andújar, E.; Pérez-Alegre, M.; Gallego-Durán, R.; Varela, L.M.; Martin-Montalvo, A.; et al. Extra virgin olive oil improved body weight and insulin sensitivity in high fat diet-induced obese LDLr−/−.Leiden mice without attenuation of steatohepatitis. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Ha, S.-K.; Chae, C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp. Anim. 2010, 59, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Jing, L.; Wang, J. A polysaccharide (PNPA) from Pleurotus nebrodensis offers cardiac protection against ischemia–reperfusion injury in rats. Carbohydr. Polym. 2015, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhang, J.; Wang, W.; Wang, X.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; Gong, Z.; et al. The Antioxidative, Antiaging, and Hepatoprotective Effects of Alkali-Extractable Polysaccharides by Agaricus bisporus. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, Â.; Barreira, J.C.M.; Antonio, A.L.; Rafalski, A.; Morales, P.; Fernández-Ruiz, V.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Gamma and electron-beam irradiation as viable technologies for wild mushrooms conservation: Effects on macro- and micro-elements. Eur. Food Res. Technol. 2016, 242, 1169–1175. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martinez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Sahin, H.; Malkoç, M. Wild Edible Mushrooms as a Natural Source of Phenolics and Antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dbass, A.M.; Daihan, S.K.A.; Bhat, R.S. Agaricus blazei Murill as an efficient hepatoprotective and antioxidant agent against CCl4-induced liver injury in rats. Saudi J. Biol. Sci. 2012, 19, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano-Aguilar, G.; Jang, S.; Lakshman, S.; Gupta, R.; Beshah, E.; Sikaroodi, M.; Vinyard, B.; Molokin, A.; Gillevet, P.; Urban, J. The Effect of Dietary Mushroom Agaricus bisporus on Intestinal Microbiota Composition and Host Immunological Function. Nutrients 2018, 10, 1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, M.; Nishitani, Y. Immunomodulating compounds in Basidiomycetes. J. Clin. Biochem. Nutr. 2013, 52, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahila, J.; Ishikawa, Y.; Ohshima, T. Effects of Ergothioneine-Rich Mushroom Extract on the Oxidative Stability of Astaxanthin in Liposomes. J. Agric. Food Chem. 2019, 67, 3491–3501. [Google Scholar] [CrossRef]
- Liu, B.; Deng, X.; Jiang, Q.; Li, G.; Zhang, J.; Zhang, N.; Xin, S.; Xu, K. Scoparone alleviates inflammation, apoptosis and fibrosis of non-alcoholic steatohepatitis by suppressing the TLR4/NF-κB signaling pathway in mice. Int. Immunopharmacol. 2019, 75. [Google Scholar] [CrossRef]
- Li, W.; Yang, G.-L.; Zhu, Q.; Zhong, X.-H.; Nie, Y.-C.; Li, X.-H.; Wang, Y. TLR4 promotes liver inflammation by activating the JNK pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7655–7662. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Z.; Shen, B.; Zhang, Q.; Feng, H. Protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-κB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2017, 45, 148–155. [Google Scholar] [CrossRef]
- Leng, W.; Liu, Y.; Shi, H.; Li, S.; Zhu, H.; Pi, D.; Hou, Y.; Gong, J. Aspartate alleviates liver injury and regulates mRNA expressions of TLR4 and NOD signaling-related genes in weaned pigs after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Uyanoğlu, M.; Canbek, M.; Van Griensven, L.J.L.D.; Yamaç, M.; Senturk, H.; Kartkaya, K.; Oğlakçı, A.; Turgak, O.; Kanbak, G. Effects of polysaccharide from fruiting bodies ofAgaricus bisporus, Agaricus brasiliensis, and Phellinus linteuson alcoholic liver injury. Int. J. Food Sci. Nutr. 2014, 65, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Kala, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological properties of Agaricus bisporus fruiting bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushroom: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.d.M.; Sánchez de Medina, V.; Bautista, J.; Priego-Capote, F.; Luque de Castro, M.D. Tentative identification of the composition of Agaricus bisporus aqueous enzymatic extracts with antiviral activity against HCV: A study by liquid chromatography–tandem mass spectrometry in high resolution mode. J. Funct. Foods 2016, 24, 403–419. [Google Scholar] [CrossRef]
- Colella, A.D.; Chegenii, N.; Tea, M.N.; Gibbins, I.L.; Williams, K.A.; Chataway, T.K. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Anal. Biochem. 2012, 430, 108–110. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, P.; Luque-Sierra, A.; Falcon, G.; Carbonero, P.; Grande, L.; Bautista, J.D.; Martín, F.; Del Campo, J.A. White Button Mushroom Extracts Modulate Hepatic Fibrosis Progression, Inflammation, and Oxidative Stress In Vitro and in LDLR-/- Mice. Foods 2021, 10, 1788. https://doi.org/10.3390/foods10081788
Gallego P, Luque-Sierra A, Falcon G, Carbonero P, Grande L, Bautista JD, Martín F, Del Campo JA. White Button Mushroom Extracts Modulate Hepatic Fibrosis Progression, Inflammation, and Oxidative Stress In Vitro and in LDLR-/- Mice. Foods. 2021; 10(8):1788. https://doi.org/10.3390/foods10081788
Chicago/Turabian StyleGallego, Paloma, Amparo Luque-Sierra, Gonzalo Falcon, Pilar Carbonero, Lourdes Grande, Juan D. Bautista, Franz Martín, and José A. Del Campo. 2021. "White Button Mushroom Extracts Modulate Hepatic Fibrosis Progression, Inflammation, and Oxidative Stress In Vitro and in LDLR-/- Mice" Foods 10, no. 8: 1788. https://doi.org/10.3390/foods10081788
APA StyleGallego, P., Luque-Sierra, A., Falcon, G., Carbonero, P., Grande, L., Bautista, J. D., Martín, F., & Del Campo, J. A. (2021). White Button Mushroom Extracts Modulate Hepatic Fibrosis Progression, Inflammation, and Oxidative Stress In Vitro and in LDLR-/- Mice. Foods, 10(8), 1788. https://doi.org/10.3390/foods10081788