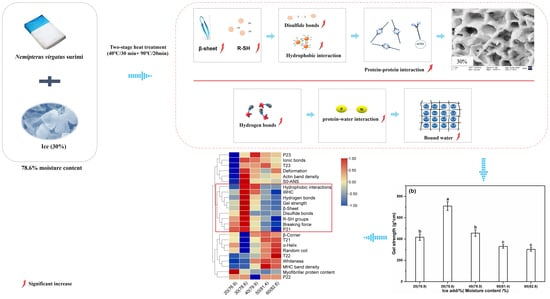
Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Breaking Force, Deformation, and Gel Strength
2.4. Water-Holding Capacity (WHC) and Whiteness
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.7. Chemical Interactions
Ionic bonds = c(SB-SA)
Hydrogen bonds = c(SC-SB)
Hydrophobic interactions = c(SD-SC)
Disulfide bonds = c(SE-SD)
2.8. Reactive Sulfhydryl (R-SH) and Surface Hydrophobicity
2.9. Myofibrillar Protein Content
2.10. Water Mobility and Distribution of Surimi Gel
2.11. Scanning Electron Microscopy (SEM)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Amount of Ice Added on the Breaking Force, Deformation, and Gel Strength of Surimi Gel
3.2. Effect of the Amount of Ice Added on the WHC and Whiteness of Surimi Gel
3.3. Effect of the Amount of Ice Added on SDS-PAGE of Surimi Gel
3.4. Effect of the Amount of Ice Added on the Secondary Structures of Surimi Gel Protein
3.5. Effect of the Amount of Ice Added on the Chemical Interactions of Surimi Gel
3.6. Effect of the Amount of Ice Added on the Content of R-SH Group and Surface Hydrophobicity of Surimi Gel
3.7. Effect of the Amount of Ice Added on the Myofibrillar Protein Content of Surimi
3.8. Effect of the Amount of Ice Added on the Water Mobility and Distribution of Surimi Gel
3.9. Effect of the Amount of Ice Added on The Microstructure of Surimi Gel
3.10. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Sun, Q.; Pan, Y.; Wei, S.; Xia, Q.; Liu, S.; Ji, H.; Deng, C.; Hao, J. Investigation on the correlation between changes in water and texture properties during the processing of surimi from golden pompano (Trachinotus ovatus). J. Food Sci. 2021, 86, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Wang, X. Effects of hydrolyzed wheat gluten on the properties of high temperature (100 °C) treated surimi gels. Food Hydrocoll. 2015, 45, 196–202. [Google Scholar] [CrossRef]
- Cando, D.; Moreno, H.M.; Tovar, C.A.; Herranz, B.; Borderias, A.J. Effect of high pressure and/or temperature over gelation of isolated hake myofibrils. Food Bioprocess Technol. 2014, 7, 3197–3207. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Li, X.; Li, R.; Yi, S.; Xu, Y.; Mi, H.; Li, J. Changes of water state and gel characteristics of hairtail (Trichiurus lepturus) surimi during thermal processing. J. Texture Stud. 2019, 50, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, K.; Ye, T.; Wan, S.; Wang, Y.; Wang, D.; Li, B.; Wang, C. Influence of konjac glucomannan on gelling properties and water state in egg white protein gel. Food Res. Int. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined use of rheology, LF-NMR, and MRI for characterizing the gel properties of hairtail surimi with potato starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Pin, M.R.; Laca, A.; Paredes, B.; Díaz, M. Blue whiting surimi paste as affected by moisture content, temperature and pH processing conditions. Rheological evaluation. Acta Aliment. 2010, 39, 445–456. [Google Scholar] [CrossRef]
- Park, H.W.; Park, J.W.; Yoon, W.B. Developing a linearization method to determine an optimum formulation in surimi blend with varied moisture content using linear programming. Int. J. Food Eng. 2017, 16, 16–24. [Google Scholar] [CrossRef]
- Esturk, O.; Park, J.W.; Baik, M.; Kim, B. Effects of moisture content on non-fracture dynamic properties and fracture quality of pacific whiting surimi. Food Sci. Biotechnol. 2006, 15, 856–859. [Google Scholar] [CrossRef]
- Reppond, K.D.; Babbitt, J.K. Gel properties of surimi from various fish species as affected by moisture content. J. Food Sci. 1997, 62, 33–36. [Google Scholar] [CrossRef]
- Benjakul, S.; Chantarasuwan, C.; Visessanguan, W. Effect of medium temperature setting on gelling characteristics of surimi from some tropical fish. Food Chem. 2003, 82, 567–574. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Q.; Zheng, Y.; Wang, J.; Tian, Y.; Zheng, B.; Guo, Z. Effect of two-step microwave heating on the gelation properties of golden threadfin bream (Nemipterus virgatus) myosin. Food Chem. 2020, 328, 127104. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; AOAC: Washington, DC, USA, 2002. [Google Scholar]
- Kocher, P.N.; Foegeding, E.A. Microcentrifuge-based method for measuring water-holding of protein gels. J. Food Sci. 1993, 5, 1040–1046. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT Food Sci. Technol. 2013, 50, 511–518. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Borderías, A.J.; Montero, P. Chemical interactions of nonmuscle proteins in the network of sardine (Sardina pilchardus) muscle gels. LWT Food Sci. Technol. 1997, 30, 602–608. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Bhandari, B.; Gao, Z. Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 2016, 86, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Park, H.W.; Lee, M.G.; Park, J.W.; Yoon, W.B. Developing an accurate heat transfer simulation model of Alaska pollock surimi paste by estimating the thermal diffusivities at various moisture and salt contents. Int. J. Food Eng. 2019, 16. [Google Scholar] [CrossRef]
- Poowakanjana, S.; Park, J.W.; Moon, J.H.; Yoon, W.B. Assessing the dynamic rheology at various frequencies of surimi paste as affected by heating pates and moisture contents. J. Texture Stud. 2015, 46, 302–311. [Google Scholar] [CrossRef]
- Park, J.W. Surimi gel colors as affected by moisture content and physical conditions. J. Food Sci. 1995, 60, 15–18. [Google Scholar] [CrossRef]
- Chen, X.; Tume, R.K.; Xiong, Y.; Xu, X.; Zhou, G.; Chen, C.; Nishiumi, T. Structural modification of myofibrillar proteins by high-pressure processing for functionally improved, value-added and healthy muscle gelled foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2981–3003. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Phatcharat, S.; Tammatinna, A.; Visessanguan, W.; Kishimura, H. Improvement of gelling properties of lizardfish mince as influenced by microbial transglutaminase and fish freshness. J. Food Sci. 2008, 73, S239–S246. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, A.; Liu, G.; Ying, D.; Xiao, J.; Miao, J. Changes of physicochemical properties of greater lizardfish (Saurida tumbil) surimi gels treated with high pressure combined with microbial transglutaminase. J. Food Process. Preserv. 2019, 43, e14150. [Google Scholar] [CrossRef]
- Imtiaz-Ul-Islam, M.; Liang, H.; Langrish, T. CO2 capture using whey protein isolate. Chem. Eng. J. 2011, 171, 1069–1081. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2010, 10, 33–51. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocoll. 2015, 51, 176–187. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Zheng, B.; Zhang, Y.; Guo, Z. Effect of ultra-high pressure on the structure and gelling properties of low salt golden threadfin bream (Nemipterus virgatus) myosin. LWT Food Sci. Technol. 2019, 100, 381–390. [Google Scholar] [CrossRef]
- Zheng, H.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of κ-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/ precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, A.; Benjakul, S.; Zou, Y.; Liu, X.; Xiao, S. The mechanism of low-level pressure coupled with heat treatment on water migration and gel properties of Nemipterus virgatus surimi. LWT Food Sci. Technol. 2021, 150, 112086. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Y.L.; Sato, H. Rheological enhancement of pork myofibrillar protein-lipid emulsion composite gels via glucose oxidase oxidation/transglutaminase cross-linking pathway. J. Agric. Food Chem. 2017, 65, 8451–8458. [Google Scholar] [CrossRef]
- Grossi, A.; Olsen, K.; Bolumar, T.; Rinnan, A.; Ogendal, L.H.; Orlien, V. The effect of high pressure on the functional properties of pork myofibrillar proteins. Food Chem. 2016, 196, 1005–1015. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Zhou, Y.; Wang, X.; Ma, J.; Xiong, G.; Wang, L.; Wang, S.; Sun, W. Gel properties of grass carp myofibrillar protein modified by low-frequency magnetic field during two-stage water bath heating. Food Hydrocoll. 2020, 107, 105920. [Google Scholar] [CrossRef]






| Ice Adds/% (Moisture Content/%) | L* | a* | b* | MHC Band Density | Actin Band Density |
|---|---|---|---|---|---|
| 20 (76.9) | 82.38 ± 0.74 b | −0.72 ± 0.16 a | 9.13 ± 0.50 a | 309.39 ± 4.24 e | 6104.26 ± 14.73 e |
| 30 (78.6) | 81.95 ± 0.50 b | −0.98 ± 0.17 b | 8.18 ± 0.18 b | 386.51 ± 14.14 d | 8241.00 ± 20.51 a |
| 40 (79.9) | 83.25 ± 0.18 a | −1.07 ± 0.12 b | 7.80 ± 0.18 bc | 636.16 ± 7.07 c | 7421.15 ± 7.98 b |
| 50 (81.4) | 83.47 ± 0.25 a | −1.03 ± 0.05 b | 7.65 ± 0.10 c | 773.47 ± 2.46 a | 7354.86 ± 28.79 c |
| 60 (82.6) | 83.08 ± 0.52 a | −1.12 ± 0.10 b | 7.22 ± 0.50 d | 751.13 ± 5.36 b | 7255.34 ± 9.60 d |
| Ice Adds/% (Moisture Content/%) | α-Helix (%) | β-Sheet (%) | β-Corner (%) | Random Coil (%) |
|---|---|---|---|---|
| 20 (76.9) | 19.77 ± 0.22 a | 19.31 ± 0.33 b | 42.79 ± 0.08 ab | 18.13 ± 0.03 a |
| 30 (78.6) | 19.17 ± 0.15 b | 20.74 ± 0.32 a | 42.37 ± 0.09 b | 17.73 ± 0.09 b |
| 40 (79.9) | 19.59 ± 0.18 ab | 19.44 ± 0.73 b | 42.90 ± 0.44 ab | 18.08 ± 0.15 a |
| 50 (81.4) | 19.79 ± 0.29 a | 19.02 ± 0.72 b | 42.96 ± 0.22 a | 18.23 ± 0.22 a |
| 60 (82.6) | 19.93 ± 0.16 a | 18.85 ± 0.36 b | 42.89 ± 0.14 ab | 18.34 ± 0.10 a |
| Ice Adds/% (Moisture Content/%) | 20 (76.9) | 30 (78.6) | 40 (79.9) | 50 (81.4) | 60 (82.6) |
|---|---|---|---|---|---|
| T21 (ms) | 0.40 ± 0.03 ab | 0.38 ± 0.00 b | 0.40 ± 0.03 ab | 0.41 ± 0.03 a | 0.41 ± 0.03 a |
| - | 0.75 ± 0.00 b | 0.80 ± 0.06 b | 0.92 ± 0.07 b | 1.45 ± 0.19 a | |
| T22 (ms) | 65.79 ± 0.00 e | 70.72 ± 0.00 d | 75.65 ± 0.00 c | 86.98 ± 0.00 b | 100 ± 0.00 a |
| T23 (ms) | 1158.57 ± 172.60 b | 2168.44 ± 301.87 a | 2180.91 ± 398.46 a | 2357.68 ± 307.66 a | 2050.59 ± 267.59 a |
| P21 (%) | 3.09 ± 0.07 ab | 3.20 ± 0.22 a | 2.88 ± 0.17 b | 2.66 ± 0.09 c | 2.41 ± 0.24 d |
| P22 (%) | 96.24 ± 0.23 b | 96.67 ± 0.19 a | 96.51 ± 0.26 ab | 96.86 ± 0.06 a | 96.87 ± 0.14 a |
| P23 (%) | 0.49 ± 0.01 ab | 0.38 ± 0.10 b | 0.57 ± 0.10 a | 0.43 ± 0.02 ab | 0.40 ± 0.08 b |
| Index | Correlation Coefficient | Index | Correlation Coefficient |
|---|---|---|---|
| Breaking force | 0.908 * | SH group | 0.900 * |
| Deformation | 0.418 | S0-ANS | 0.717 |
| WHC | 0.912 * | T21 | −0.994 ** |
| Whiteness | −0.650 | T22 | −0.647 |
| α-Helix | −0.983 ** | T23 | 0.071 |
| β-Sheet | 0.996 ** | P21 | 0.824 |
| β-Corner | −0.936 * | P22 | −0.047 |
| Random coil | −0.994 ** | P23 | 0.021 |
| Ionic bands | 0.402 | MHC band density | −0.725 |
| Hydrogen bonds | 0.974 ** | Actin band density | 0.602 |
| Hydrophobic interactions | 0.784 | Myofibrillar protein content | 0.097 |
| Disulfide bonds | 0.920 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zou, Y.; Zhou, A.; Xiao, J.; Benjakul, S. Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration. Foods 2021, 10, 1815. https://doi.org/10.3390/foods10081815
Chen H, Zou Y, Zhou A, Xiao J, Benjakul S. Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration. Foods. 2021; 10(8):1815. https://doi.org/10.3390/foods10081815
Chicago/Turabian StyleChen, Haiqiang, Yiqian Zou, Aimei Zhou, Jie Xiao, and Soottawat Benjakul. 2021. "Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration" Foods 10, no. 8: 1815. https://doi.org/10.3390/foods10081815
APA StyleChen, H., Zou, Y., Zhou, A., Xiao, J., & Benjakul, S. (2021). Insight into the Effect of Ice Addition on the Gel Properties of Nemipterus virgatus Surimi Gel Combined with Water Migration. Foods, 10(8), 1815. https://doi.org/10.3390/foods10081815