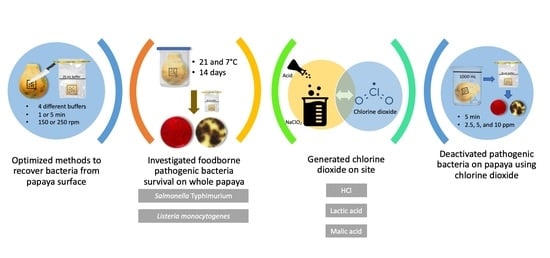
Fate of Salmonella Typhimurium and Listeria monocytogenes on Whole Papaya during Storage and Antimicrobial Efficiency of Aqueous Chlorine Dioxide Generated with HCl, Malic Acid or Lactic Acid on Whole Papaya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cell Cultures
2.2. Preparation of Papayas and Inocula
2.3. Optimization of Recovery Method for Counting Bacteria Cells on Papaya Surface
2.3.1. Recovery Method
2.3.2. PH of Papaya Skin Homogenate as Affected by Homogenization Parameters
2.4. Behavior of Pathogenic Bacteria on Whole Papayas Stored at Different Temperatures
2.5. ClO2 Treatment on Whole Papayas
2.5.1. Preparation of Aqueous ClO2
2.5.2. Washing Papayas with Aqueous ClO2 and Individual Acid Solutions
2.5.3. ClO2 Residue on Papaya Surface after Washing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Recovery of S. Typhimurium and L. monocytogenes Cells from Whole Papaya Surface as Affected by Homogenization Parameters and Enumeration Methods
3.2. Behavior of Pathogenic Bacteria on Whole Papayas Stored at Different Temperatures
3.3. Inactivation of S. Typhimurium and L. monocytogenes on Whole Papayas Using Aqueous ClO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Evans, E.A.; Ballen, F.H.; Crane, J.H. An Overview of US Papaya Production, Trade, and Consumption; Electronic Data Information Source (EDIS) FE914; University of Florida: Gainesville, FL, USA, 2012; Volume 9, pp. 1–8. [Google Scholar]
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 23 May 2021).
- Gibbs, R.; Pingault, N.; Mazzucchelli, T.; O’Reilly, L.; MacKenzie, B.; Green, J.; Mogyorosy, R.; Stafford, R.; Bell, R.; Hiley, L.; et al. An Outbreak of Salmonella enterica Serotype Litchfield Infection in Australia Linked to Consumption of Contaminated Papaya. J. Food Prot. 2009, 72, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.; Whitney, B.; Williams, D.L.; Holloman, K.; Grady, D.; Thomas, D.; Omoregie, E.; Lamba, K.; Leeper, M.; Gieraltowski, L.; et al. Multistate outbreaks of Salmonella infections linked to imported Maradol papayas—United States, December 2016–September 2017. Epidemiol. Infect. 2019, 147, e265. [Google Scholar] [CrossRef] [Green Version]
- FDA. Letter to Papaya Grower, Harvesters, Packers, Distributors, Exporters, Importers, and Retailers Concerning Food-Borne Illness Outbreaks Tied to Papayas. 2019. Available online: https://www.fda.gov/media/130271/download (accessed on 23 May 2021).
- de Oliveira, J.G.; Vitória, A.P. Papaya: Nutritional and pharmacological characterization, and quality loss due to physiological disorders. An overview. Food Res. Int. 2011, 44, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Strawn, L.K.; Schneider, K.R.; Danyluk, M.D. Microbial Safety of Tropical Fruits. Crit. Rev. Food Sci. Nutr. 2011, 51, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Marik, C.M.; Zuchel, J.; Schaffner, D.W.; Strawn, L.K. Growth and Survival of Listeria monocytogenes on Intact Fruit and Vegetable Surfaces During Postharvest Handling: A Systematic Literature Review. J. Food Prot. 2019, 83, 108–128. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms. Colorado|Listeria|CDC. 2012. Available online: https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html (accessed on 23 May 2021).
- CDC. Multistate Outbreak of Listeriosis Linked to Commercially Produced, Prepackaged Caramel Apples Made from Bidart Bros. Apples|Listeria|CDC. 2015. Available online: https://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/index.html (accessed on 23 May 2021).
- Poimenidou, S.V.; Chatzithoma, D.-N.; Nychas, G.-J.; Skandamis, P.N. Adaptive Response of Listeria monocytogenes to Heat, Salinity and Low pH, after Habituation on Cherry Tomatoes and Lettuce Leaves. PLoS ONE 2016, 11, e0165746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel-Vargas, E.; Luna-Rojo, A.M.; Cadena-Ramírez, A.; Torres-Vitela, R.; Gomez-Aldapa, C.A.; Villarruel-López, A.; Téllez-Jurado, A.; Villagómez-Ibarra, J.R.; Reynoso-Camacho, R.; Castro-Rosas, J. Behavior of 11 Foodborne Bacteria on Whole and Cut Mangoes var. Ataulfo and Kent and Antibacterial Activities of Hibiscus sabdariffa Extracts and Chemical Sanitizers Directly onto Mangoes Contaminated with Foodborne Bacteria. J. Food Prot. 2018, 81, 743–753. [Google Scholar] [CrossRef]
- Sheng, L.; Edwards, K.; Tsai, H.-C.; Hanrahan, I.; Zhu, M.-J. Fate of Listeria monocytogenes on Fresh Apples under Different Storage Temperatures. Front. Microbiol. 2017, 8, 1396. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Wang, L. Survival of Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes on Fresh and Sliced Green and Golden Kiwifruits. Foodborne Pathog. Dis. 2018, 15, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hu, W.; Jiang, A.; Xu, Y.; Sarengaowa; Li, X.; Bai, X. Growth Potential of Listeria Monocytogenes and Staphylococcus Aureus on Fresh-Cut Tropical Fruits. J. Food Sci. 2015, 80, M2548–M2554. [Google Scholar] [CrossRef] [PubMed]
- Penteado, A.L.; Leitão, M.F. Growth of Listeria monocytogenes in melon, watermelon and papaya pulps. Int. J. Food Microbiol. 2004, 92, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Penteado, A.L.; Leitão, M.F. Growth of Salmonella Enteritidis in melon, watermelon and papaya pulp stored at different times and temperatures. Food Control 2004, 15, 369–373. [Google Scholar] [CrossRef]
- Strawn, L.K.; Danyluk, M.D. Fate of Escherichia coli O157:H7 and Salmonella spp. on fresh and frozen cut mangoes and papayas. Int. J. Food Microbiol. 2010, 138, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yemmireddy, V. Fate of Salmonella spp. in fresh-cut papaya (Carica papaya L.) at different storage temperature and relative humidity. LWT 2021, 148, 111810. [Google Scholar] [CrossRef]
- Huff, K.; Boyer, R.; Denbow, C.; O’Keefe, S.; Williams, R. Effect of Storage Temperature on Survival and Growth of Foodborne Pathogens on Whole, Damaged, and Internally Inoculated Jalapeños (Capsicum annuum var. annuum). J. Food Prot. 2012, 75, 382–388. [Google Scholar] [CrossRef]
- Penteado, A.L.; Eblen, B.S.; Miller, A.J. Evidence of Salmonella Internalization into Fresh Mangos during Simulated Postharvest Insect Disinfestation Procedures. J. Food Prot. 2004, 67, 181–184. [Google Scholar] [CrossRef]
- Perez-Rodriguez, F.; Begum, M.; Johannessen, G. Study of the cross-contamination and survival of Salmonella in fresh apples. Int. J. Food Microbiol. 2014, 184, 92–97. [Google Scholar] [CrossRef]
- Tadepalli, S.; Bridges, D.F.; Driver, R.; Wu, V.C. Effectiveness of different antimicrobial washes combined with freezing against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on blueberries. Food Microbiol. 2018, 74, 34–39. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. CFR173.300. CFR—Code of Federal Regulations Title 21. 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=173.300 (accessed on 23 May 2021).
- Gómez-López, V.M.; Rajkovic, A.; Ragaert, P.; Smigic, N.; Devlieghere, F. Chlorine dioxide for minimally processed produce preservation: A review. Trends Food Sci. Technol. 2009, 20, 17–26. [Google Scholar] [CrossRef]
- Van Haute, S.; Tryland, I.; Escudero, C.; Vanneste, M.; Sampers, I. Chlorine dioxide as water disinfectant during fresh-cut iceberg lettuce washing: Disinfectant demand, disinfection efficiency, and chlorite formation. LWT 2017, 75, 301–304. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#one (accessed on 11 July 2021).
- Adhikari, A.; Chhetri, V.; Bhattacharya, D.; Cason, C.; Luu, P.; Suazo, A. Effectiveness of daily rinsing of alfalfa sprouts with aqueous chlorine dioxide and ozonated water on the growth of Listeria monocytogenes during sprouting. Lett. Appl. Microbiol. 2019, 69, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Banach, J.; van Overbeek, L.; Groot, M.N.; van der Zouwen, P.; van der Fels-Klerx, H. Efficacy of chlorine dioxide on Escherichia coli inactivation during pilot-scale fresh-cut lettuce processing. Int. J. Food Microbiol. 2018, 269, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ni Tan, J.; Hwang, C.-A.; Huang, L.; Wu, V.C.H.; Hsiao, H.-I. In Situ Generation of Chlorine Dioxide for Decontamination of Salmonella, Listeria monocytogenes, and Pathogenic Escherichia coli on Cantaloupes, Mung Beans, and Alfalfa Seeds. J. Food Prot. 2020, 83, 287–294. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Han, Z. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L.). J. Biosci. Bioeng. 2011, 111, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. Evaluation of Chlorine Dioxide Gas Residues on Selected Food Produce. J. Food Sci. 2010, 76, T11–T15. [Google Scholar] [CrossRef] [PubMed]
- Boonyaritthongchai, P.; Techavuthiporn, C.; Cumsingnok, T. Effect of Acidified Sodium Chlorite and Packaging on Microbial Reduction and Quality Maintenance of Shredded Green Papaya; Acta Hortic: Istanbul, Turkey, 2018; Volume 1292, pp. 287–292. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C. Effects of ozone on major antioxidants and microbial populations of fresh-cut papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Gu, G.; Bolten, S.; Mendes-Oliveira, G.; Zhou, B.; Teng, Z.; Pearlstein, D.; Luo, Y.; Millner, P.; Nou, X. Salmonella inactivation and sponge/microfiber mediated cross-contamination during papaya wash with chlorine or peracetic acid as sanitizer. Food Microbiol. 2020, 95, 103677. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine Dioxide: The Current State of the Art. Ozone Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y.; Beuchat, L.R.; Ryu, J.-H. Production and stability of chlorine dioxide in organic acid solutions as affected by pH, type of acid, and concentration of sodium chlorite, and its effectiveness in inactivating Bacillus cereus spores. Food Microbiol. 2008, 25, 964–969. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y. Comparison of aqueous chlorine dioxide generated with different acids on reducing foodborne pathogenic bacteria. In Proceedings of the International Association for Food Protection 2020 Annual Meeting, Des Moines, IA, USA, 26–28 October 2020. [Google Scholar]
- Lang, M.M.; Harris, L.J.; Beuchat, L.R. Evaluation of Inoculation Method and Inoculum Drying Time for Their Effects on Survival and Efficiency of Recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes Inoculated on the Surface of Tomatoes. J. Food Prot. 2004, 67, 732–741. [Google Scholar] [CrossRef]
- Kim, S.-R.; Yoon, Y.; Kim, W.-I.; Park, K.-H.; Yun, H.-J.; Chung, D.H.; Yun, J.C.; Ryu, K.Y. Comparison of Sample Preparation Methods for the Recovery of Foodborne Pathogens from Fresh Produce. J. Food Prot. 2012, 75, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Suzuki, J.Y.; Carr, J.B.; McQuate, G.T.; Ferreira, S.A.; Manshardt, R.M.; Pitz, K.Y.; Wall, M.M.; Gonsalves, D. Nutritional composition of Rainbow papaya, the first commercialized transgenic fruit crop. J. Food Compos. Anal. 2011, 24, 140–147. [Google Scholar] [CrossRef]
- Collignon, S.; Korsten, L. Attachment and Colonization by Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica subsp. enterica serovar Typhimurium, and Staphylococcus aureus on Stone Fruit Surfaces and Survival through a Simulated Commercial Export Chain. J. Food Prot. 2010, 73, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.H. A review of microbial injury and recovery methods in food. Food Microbiol. 2008, 25, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Paull, R.E.; Chen, N.J. Papaya: Postharvest Quality-Maintenance Guidelines. Fruit, Nut, and Beverage Crops; UH–CTAHR Extension Publication: Honolulu, HI, USA, 2014; Volume 34, pp. 2–4. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/F_N-34.pdf (accessed on 23 May 2021).
- Visvalingam, J.; Holley, R.A. Evaluation of chlorine dioxide, acidified sodium chlorite and peroxyacetic acid for control of Escherichia coli O157:H7 in beef patties from treated beef trim. Food Res. Int. 2018, 103, 295–300. [Google Scholar] [CrossRef]
- Wu, V.C.; Rioux, A. A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage. Food Microbiol. 2010, 27, 179–184. [Google Scholar] [CrossRef]
- Brandl, M.T.; Huynh, S. Effect of the Surfactant Tween 80 on the Detachment and Dispersal of Salmonella enterica Serovar Thompson Single Cells and Aggregates from Cilantro Leaves as Revealed by Image Analysis. Appl. Environ. Microbiol. 2014, 80, 5037–5042. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yu, Q.; Shao, L.; Li, X.; Dai, R. Sublethal injury and recovery of Escherichia coli O157:H7 after ohmic heating. Food Control 2018, 94, 85–92. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zhong, Q. Quality attributes and microbial survival on whole cantaloupes with antimicrobial coatings containing chitosan, lauric arginate, cinnamon oil and ethylenediaminetetraacetic acid. Int. J. Food Microbiol. 2016, 235, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koseki, S.; Nakamura, N.; Shiina, T. Comparison of Desiccation Tolerance among Listeria monocytogenes, Escherichia coli O157:H7, Salmonella enterica, and Cronobacter sakazakii in Powdered Infant Formula. J. Food Prot. 2015, 78, 104–110. [Google Scholar] [CrossRef]
- Dhowlaghar, N.; Tang, J.; Zhu, M.-J. Thermal inactivation of Salmonella, Listeria monocytogenes and Enterococcus faecium NRRL B-2354 in desiccated shredded coconut. LWT 2021, 149, 111851. [Google Scholar] [CrossRef]
- Mathew, E.N.; Muyyarikkandy, M.S.; Kuttappan, D.; Amalaradjou, M.A. Attachment of Salmonella enterica on Mangoes and Survival Under Conditions Simulating Commercial Mango Packing House and Importer Facility. Front. Microbiol. 2018, 9, 1519. [Google Scholar] [CrossRef]
- Bardsley, C.A.; Truitt, L.N.; Pfuntner, R.C.; Danyluk, M.D.; Rideout, S.L.; Strawn, L.K. Growth and Survival of Listeria monocytogenes and Salmonella on Whole and Sliced Cucumbers. J. Food Prot. 2019, 82, 301–309. [Google Scholar] [CrossRef]
- Behrsing, J.; Jaeger, J.; Horlock, F.; Kita, N.; Franz, P.; Premier, R. Survival of Listeria innocua, Salmonella salford and Escherichia coli on the surface of fruit with inedible skins. Postharvest Biol. Technol. 2003, 29, 249–256. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Gomez-Aldapa, C.; Acevedo-Sandoval, O.A.; Ramírez, C.A.G.; Villagomez-Ibarra, J.R.; Hernández, N.C.; Villarruel-Lopez, A.; Torres-Vitela, M.D.R. Frequency and Behavior of Salmonella and Escherichia coli on Whole and Sliced Jalapeño and Serrano Peppers. J. Food Prot. 2011, 74, 874–881. [Google Scholar] [CrossRef]
- Knudsen, D.M.; Yamamoto, S.A.; Harris, L.J. Survival of Salmonella spp. and Escherichia coli O157:H7 on Fresh and Frozen Strawberries. J. Food Prot. 2001, 64, 1483–1488. [Google Scholar] [CrossRef]
- Salazar, J.K.; Carstens, C.K.; Bathija, V.M.; Narula, S.S.; Parish, M.; Tortorello, M.L. Fate of Listeria monocytogenes in Fresh Apples and Caramel Apples. J. Food Prot. 2016, 79, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wiedmann, M. Physiology and Genetics of Listeria Monocytogenes Survival and Growth at Cold Temperatures. Crit. Rev. Food Sci. Nutr. 2008, 49, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.L.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A Comparison of Different Chemical Sanitizers for Inactivating Escherichia coli O157:H7 and Listeria monocytogenes in Solution and on Apples, Lettuce, Strawberries, and Cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Bridges, D.F.; Anderson, R.; Zhang, R.; Wu, V.C. Synergistic effect of sequential wash treatment with two different low-dosage antimicrobial washes in combination with frozen storage increases Salmonella Typhimurium and Listeria monocytogenes reduction on wild blueberries. Food Control 2019, 102, 87–93. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella Typhimurium. LWT 2016, 66, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.; Pohlman, F. Role of organic acids and peroxyacetic acid as antimicrobial intervention for controlling Escherichia coli O157:H7 on beef trimmings. LWT 2016, 65, 868–873. [Google Scholar] [CrossRef]
- EPA. Reregistration Eligibility Decision (RED) for Chlorine Dioxide and Sodium Chlorite (Case 4023). 2006. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-020503_3-Aug-06.pdf (accessed on 23 May 2021).
- Mathew, E.N.; Muyyarikkandy, M.S.; Bedell, C.; Amalaradjou, M.A. Efficacy of Chlorine, Chlorine Dioxide, and Peroxyacetic Acid in Reducing Salmonella Contamination in Wash Water and on Mangoes Under Simulated Mango Packinghouse Washing Operations. Front. Sustain. Food Syst. 2018, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Pao, S.; Kelsey, D.F.; Long, W. Spray washing of tomatoes with chlorine dioxide to minimize Salmonella on inocu-lated fruit surfaces and cross-contamination from revolving brushes†. J. Food Prot. 2009, 72, 2448–2452. [Google Scholar] [CrossRef]


| Buffer | 1 Min | 5 Min | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 Rpm | 250 Rpm | 150 Rpm | 250 Rpm | ||||||
| XLD | PCA + XLD | XLD | PCA + XLD | XLD | PCA + XLD | XLD | PCA + XLD | ||
| PBS | 5.31 ± 0.65 | 5.34 ± 0.84 | 4.91 ± 0.38 | 4.95 ± 0.44 | 5.14 ± 0.29 | 5.38 ± 0.18 | 4.86 ± 0.82 | 4.81 ± 0.99 | 5.11 ± 0.57 a,b |
| PEPT | 4.77 ± 0.50 | 4.80 ± 0.43 | 4.99 ± 0.52 | 5.05 ± 0.46 | 4.57 ± 0.47 | 4.85 ± 0.67 | 4.74 ± 0.32 | 4.54 ± 0.22 | 4.77 ± 0.40 b |
| PBS + T | 5.08 ± 0.26 | 5.18 ± 0.30 | 5.43 ± 0.38 | 5.55 ± 0.34 | 5.64 ± 0.46 | 5.39 ± 0.49 | 5.29 ± 0.10 | 5.31 ± 0.07 | 5.36 ± 0.33 a |
| PEPT + T | 5.08 ± 0.87 | 5.25 ± 0.74 | 5.26 ± 0.47 | 5.45 ± 0.41 | 5.07 ± 0.34 | 5.49 ± 0.28 | 5.39 ± 0.50 | 5.41 ± 0.24 | 5.28 ± 0.45 a |
| Buffer | 1 Min | 5 Min | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 Rpm | 250 Rpm | 150 Rpm | 250 Rpm | ||||||
| MOX | PCA + MOX | MOX | PCA + MOX | MOX | PCA + MOX | MOX | PCA + MOX | ||
| PBS | 4.23 ± 0.49 | 4.49 ± 0.75 | 4.79 ± 0.17 | 4.76 ± 0.20 | 4.60 ± 0.81 | 4.50 ± 0.74 | 4.36 ± 0.58 | 4.35 ± 0.59 | 4.51 ± 0.52 b |
| PEPT | 5.02 ± 0.21 | 4.55 ± 0.62 | 4.84 ± 0.55 | 4.67 ± 0.45 | 4.89 ± 0.76 | 4.30 ± 0.37 | 4.57 ± 0.60 | 4.61 ± 0.45 | 4.68 ± 0.49 a,b |
| PBS + T | 4.97 ± 0.29 | 4.61 ± 0.33 | 4.97 ± 0.13 | 4.93 ± 0.15 | 4.92 ± 0.48 | 5.09 ± 0.19 | 5.08 ± 0.3 | 4.94 ± 0.01 | 4.94 ± 0.25 a |
| PEPT + T | 4.96 ± 0.63 | 4.54 ± 1.03 | 4.85 ± 0.58 | 4.88 ± 0.55 | 4.62 ± 0.83 | 4.38 ± 0.89 | 4.55 ± 1.04 | 4.49 ± 0.97 | 4.66 ± 0.73 a,b |
| Buffer | 1 Min | 5 Min | Average | ||
|---|---|---|---|---|---|
| 150 Rpm | 250 Rpm | 150 Rpm | 250 Rpm | ||
| PBS | 7.19 ± 0.08 | 7.20 ± 0.09 | 7.21 ± 0.06 | 7.22 ± 0.09 | 7.21 ± 0.07 a |
| PEPT | 6.32 ± 0.07 | 6.19 ± 0.28 | 6.37 ± 0.25 | 6.18 ± 0.05 | 6.26 ± 0.18 b |
| PBS + T | 7.11 ± 0.06 | 7.08 ± 0.06 | 7.44 ± 0.56 | 7.12 ± 0.06 | 7.19 ± 0.29 a |
| PEPT + T | 5.88 ± 0.27 | 5.87 ± 0.21 | 5.79 ± 0.06 | 6.03 ± 0.23 | 5.89 ± 0.20 c |
| Water | 6.05 ± 0.22 | 6.03 ± 0.17 | 6.10 ± 0.14 | 5.82 ± 0.08 | 6.00 ± 0.17 c |
| Acid | S. Typhimurium | L. monocytogenes |
|---|---|---|
| Water | 2.41 ± 0.24 | 3.86 ± 0.09 |
| HCl | 3.01 ± 0.42 | 3.58 ± 0.19 |
| Lactic acid | 2.77 ± 0.18 | 3.64 ± 0.43 |
| Malic acid | 2.45 ± 0.15 | 3.91 ± 0.43 |
| Acid Used to Generate ClO2 | Concentration of ClO2 Wash Solution | ||
|---|---|---|---|
| 5 ppm | 10 ppm | 20 ppm | |
| HCl | 10−3 | 10−7 | 10−7 |
| Lactic acid | 10−7 | 10−4 | 10−3 |
| Malic acid | 10−7 | 10−7 | 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Li, Y. Fate of Salmonella Typhimurium and Listeria monocytogenes on Whole Papaya during Storage and Antimicrobial Efficiency of Aqueous Chlorine Dioxide Generated with HCl, Malic Acid or Lactic Acid on Whole Papaya. Foods 2021, 10, 1871. https://doi.org/10.3390/foods10081871
Dong L, Li Y. Fate of Salmonella Typhimurium and Listeria monocytogenes on Whole Papaya during Storage and Antimicrobial Efficiency of Aqueous Chlorine Dioxide Generated with HCl, Malic Acid or Lactic Acid on Whole Papaya. Foods. 2021; 10(8):1871. https://doi.org/10.3390/foods10081871
Chicago/Turabian StyleDong, Lianger, and Yong Li. 2021. "Fate of Salmonella Typhimurium and Listeria monocytogenes on Whole Papaya during Storage and Antimicrobial Efficiency of Aqueous Chlorine Dioxide Generated with HCl, Malic Acid or Lactic Acid on Whole Papaya" Foods 10, no. 8: 1871. https://doi.org/10.3390/foods10081871
APA StyleDong, L., & Li, Y. (2021). Fate of Salmonella Typhimurium and Listeria monocytogenes on Whole Papaya during Storage and Antimicrobial Efficiency of Aqueous Chlorine Dioxide Generated with HCl, Malic Acid or Lactic Acid on Whole Papaya. Foods, 10(8), 1871. https://doi.org/10.3390/foods10081871