3.1. Load Bearing Ability in the Range of Large-Scale Deformation
Load bearing ability was first evaluated by analyzing the relationships between load and displacement in the range of pre-failure deformation. Texture softening induced by the heat was examined by comparing the changes observed in the compression curves corresponding to the cooked samples with respect to the fresh (untreated) ones.
Figure 1 shows the compression curves corresponding to the fresh and 25-min heated
Cheddar cauliflower under boiling (
B), steaming (
S) and
sous-vide (
SV).
Similar trends were observed after 10 min of heating, while no meaningful load-displacement signals were registered after 40 min of heating under boiling due to the extreme texture softening (data not showed). Previous researchers [
30] obtained similar load-displacement curves in studies focused on the effects of in-tap water boiling (95 °C) on cauliflower firmness. As can be inferred from
Figure 1, no yield point was detected. The curves showed an upward concavity over the full-range of the experienced deformation (0.00–0.50). Preliminary tests based on cyclic loading-unloading steps (data not showed) confirmed that a reversible stress contribute must be considered as a result of the increase of the cauliflower surface in contact with the instrumental plate during the early step of compression (<0.15). However, the mechanical behavior observed in the successive steps of compression (>0.15) suggested an elastoplastic structure of cauliflower organs that was characterized by strain-hardening capacity, as suggested by the increasing in load bearing ability with increasing deformation. Area under load-displacement curves (referred as to “work-to-deformation”) as well as the degree of concavity in the load-displacement curve (referred as to “strain-hardening capacity”) were considered the result of simultaneous mechanisms occurring under mechanical deformation: (i) increase of resistance (stress) of the elastic and viscoelastic elements present in the cell wall, all counterbalancing the increase of turgor pressure, and (ii) new formation of cross links and entanglements in the middle lamella, causing loss in porosity, increase of cell-to-cell cohesivity and tissue toughening. As reviewed by Cosgrove [
31], resistance to deformation due to a pre-stress in the plane of the cell wall as well as the resistance stemming from outward out-of-plane force of turgor pressure when the wall is sufficiently pressed into the cell can be considered potential factors explaining the non-linear stress-strain behavior and strain hardening response in plant cells under uniaxial deformation.
Texture softening induced by heat on a macroscopic scale was also evaluated using stiffness and strain energy release as two structure-related descriptors. stiffness (N/mm) measures the resistance to the deformation and was calculated as ratio between the load (N) corresponding to 0.5-level deformation (reached at the maximum displacement) and the maximum displacement (mm); then, it was used as a descriptor of the strain-hardening capacity. Strain energy release (N∙mm) was calculated as numerical integration of load, displacement data from preload to 0.5-level deformation, and then it was used as descriptor of the work-to-deformation. Decreases in strain energy release as induced by heat was associated with the cumulative damage caused in cell membrane (loss of turgor), cell wall (cell opening), and middle lamella (cell-to-cell separation). Decreasing in stiffness as induced by heat was associated with the damage caused in the cell membrane (loss of turgor) and middle lamella, mainly due to the pectin degradation, causing cell separation. As reviewed by Jarvis et al. [
29], thermally induced β-elimination reaction, which occurs during the cooking of vegetables, is a relatively specific method of depolymerizing pectic galacturonans esterified on the galacturonoyl carboxyl group with no known effect on other covalent bonds within the plant cell wall. During cooking, it is commonly accompanied by chelation of divalent cations by organic acids released from within the cell, but it is not normally sufficient to induce cell separation after the cells are dead and the mechanical stress induced by turgor pressure is partly or completely loss as induced by heat.
Figure 2 shows the strain energy release and stiffness levels corresponding to the fresh and 25 min heated
Cheddar samples under B, S, and SV conditions.
According to the strain energy release parameter, 25 min of heating under S and SV caused an “equivalent thermal effect” (differences were not significant, p < 0.05). However, the residual texture after S and SV was significantly lower (about 75%) than the initial level (fresh samples) and significantly higher (about 85%) than after boiling. Furthermore, 25 min of heating under boiling caused the loss of about 92% of the mechanical resistance at 0.5 deformation as compared to the fresh cauliflower. According to the stiffness parameter, equivalent thermal effects were not observed among cooking treatments: sous-vide steamed samples showed the highest residual stiffness among the cooked samples, while direct steamed samples showed an intermediate level between sous-vide steamed and boiled ones. Conversely, cauliflower boiled in water was characterized by the lowest level in the strain energy release and stiffness parameters among the cooked samples.
Because direct steamed and
sous-vide steamed samples were characterized by an equivalent strain energy release (intermediate between boiled and untreated samples) and at the same time by different stiffness levels, it is suggested that the different cooking methods may induce the same extent of softening on a macroscopic scale through two independent disassembling mechanisms that occur on a microscopic scale, i.e., cell wall separation and cell wall rupture. Changes in rheological properties in the range of large-scale deformation, reported in
Figure 1 and
Figure 2, were also compared to the corresponding temperature profiles experienced by the samples under heating.
As referred in Jarvis [
29], as soon as the integrity of the cell membrane is lost during heating, solutes of low-molecular weight can be released from the cells; however, the pore size of native cell walls is small enough to prevent the exit of macromolecules above about 10–20 kD and inhibit the access of digestive enzymes (e.g., lipases and amylases) that are larger than this size. Fractured cells will release their contents, and cells that remain intact may also do so if the pore diameter of their cell walls increases, for example due to pectin degradation.
Since the question of whether cell walls lose (rupture) or separate as induced by heat is relevant to the softening rate and location of intracellular macromolecules decompartmentalization. The effectiveness of the cell-wall microstructure in resisting these two kinds of disassembling mechanisms during cooking was evaluated in this work by analyzing the rheological properties on a cell scale size.
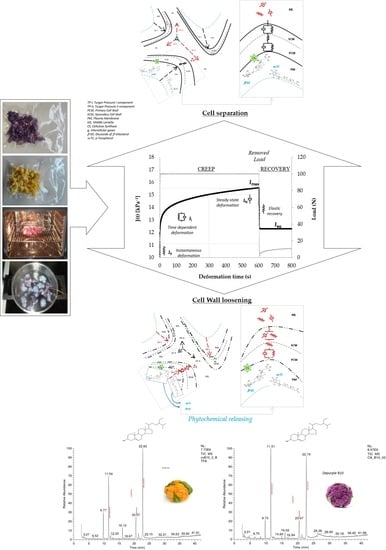
3.2. Modeling Creep Compliance at a Cellular Scale
Due to their intrinsic low sensitivity in load and deformation, large-scale deformation data cannot be suitable for assessment of the contribute of the basic elements of tissue microstructure, which include cellulose, hemicellulose, and pectin assemblies, to the tissue decompartmentalization as caused by the heat. As reviewed by Cosgrove [
31], due to the wall anisotropic biosynthesis in growing cells and to the complexity of microstructure at a local scale, wall mechanics will necessitate recognition of at least three distinct components of the volumetric elastic moduli as measurable along with the three orthogonal directions of length, with and thickness of a cell strip under micro-indentation experiments. For this reason, creep and recovery test were conducted in the range of small-scale deformation.
Figure 3 shows the time-dependent compliance, namely J(t), registered during creep and recovery test of the
Cheddar cauliflower after 25 min of steaming.
The curve was obtained by converting of displacement (mm) into compliance (kPa−1) data: J(t) was considered as the reciprocal of the uniaxial component of the volumetric elastic modulus (kPa) or, equivalently, the ratio between deformation (adimensional) and applied constant stress (kPa). As can be argued from the figure, changes in creep compliance registered during the loading step taken place through three distinguishable rheological responses. Quasi-instantaneous increasing compliance (J0) is followed by retarded relaxation (Ji) occurring for about 200–300 s, increasing toward steady-state regime (JN) before to reach the maximum level (Jmax). The equilibrium deformation (Jeq) as determined at the end of the recovery step was associated with the extent of irreversible damage caused by the applied load. The temporal succession of the compliance responses corroborated the idea that the cauliflower tissues are characterized by an elastoplastic structure at cell scale and that elastic, viscoelastic and viscoplastic relaxation mechanisms are involved to determine their compliance under external loading at different levels of the structure hierarchy. This means that the cell structural elements interact hierarchically and additively, all contributing to counterbalance both the intracellular turgor pressure and external stress through a wide spectrum of relaxation mechanisms.
Based on stress relaxation and creep experiments, several studies have shown that mechanical properties of the plant tissues at a macroscopic scale originate from the cell wall hierarchical organization both at nano- and microstructural scales [
32,
33,
34]. As reviewed by Cosgrove [
31], cellulose microfibril is the main elastic load-bearing component, while pectin plays a plastic role decreasing modulus and modifying extensibility of cellulose fibril assemblies. Again, the presence of pectin polymers in the cellulose matrix reduces its elastic properties in proportion of their content, while xyloglucans incorporation in cellulose matrix or in a cellulose-pectin matrix leads to a compliant material characterized by time-dependent (viscoelastic) creep behavior.
In this work, the overall creep compliance profile registered in the range of small-scale deformation before and after cooking was considered as a fingerprinting of the load-bearing ability of the cauliflower tissue microstructure, to which the basic elements of the cell wall contribute hierarchically and additively through elastic, viscoelastic and viscoplastic relaxation mechanisms. With the aim to evaluate the effectiveness of the main cell components in resisting cell-to-cell separation and cell wall loosening mechanisms as affected by heating, an elastoplastic mechanical model was proposed to describe quantitatively the creep behavior. Such a model was based on a hypothesis of structure-function relationships of the supramolecular assemblies among cellulose, hemicellulose and pectin and it was conceptualized by accounting for the manner that they appear interconnected in the cell wall and middle lamella microdomains as widely documented in literature. Excellent reviews and detailed papers have been published over the last two decades focused on polymer composition and hierarchical interconnection on a supramolecular scale in plant cell walls and middle lamella as well as on their mechanical properties. As reviewed by Cosgrove, Ranganathan et al., and Jarvis [
1,
29,
31], the microstructure of the primary cell wall of vegetable tissues can be envisaged as four structurally independent but interacting polymeric domains. The first domain consists of parallel layers and lamellae of cellulose microfibril (linear chains with β-(1,4) covalent bonds among glucosyl residues) with crystalline and amorphous regions that are assembled in a second domain, a three-dimensional network of hemicellulose (consisting of xyloglucans and acidic arabinoxylans). Hemicelluloses are linked to cellulose microfibrils through hydrogen bonding, with evidence for covalent linkage for arabinoxylans to cellulose through diferuloylesters that plays a role in microfibril spacing and that provides shape and strength to the cell wall. Cellulose-hemicellulose network is embedded in a third domain, the middle lamella, consisting of water-soluble calcium-binding pectin (about 30% of the dry matter of the primary cell wall), which can be considered as an extension of the previous domains from which the cellulose microfibril and calcium are lacking. Pectin is a hydrated gel-type matrix of heteropolysaccharides rich in D-galacturonic acid, including neutral sidechains of polysaccharides, consisting of linear homogalacturonan and branched blocks of rhamnogalacturonans. The cementing function of pectic substances among the individual cells depends mainly on their non-methyl esterified homogalacturonan regions, which form “egg-box” structures through ionic binding with bivalent ions (mainly calcium). There is evidence that pectin may be also covalently cross-linked through covalent bonds to phenolic acids and hemicelluloses by means of ferulic acid dimers. The fourth domain of cell wall structure consists of structural glycoproteins, oriented radially within the primary cell wall network, of which are water soluble and with lubrication (viscous and plastic) properties.
Scheme 2 illustrates the hypothesis relationships between mechanical analog elements in the proposed model, pictured as a tailored Burger’s array, and cell polymer assemblies at cell wall and middle lamella microdomains.
Each mechanical element in the model was here associated with specific supramolecular assembly of cellulose, hemicellulose and pectin interacting additively at distinct levels of the microstructure hierarchy. One in-series independent viscoelastic spring-dashpot arrays (Voigt element,
J1), describing the first component of the time-dependent reversible compliance in the model, was associated with the entangled network assemblies of cellulose microfibril, hemicelluloses, and pectin located in the secondary cell wall, the young part of the cell wall. The lack of an elastic spring in such a microdomain was justified by the high proportion of meristematic cells in the cauliflower under investigation. One
in-series independent spring (elastic component of the Maxwell element,
J0), describing the reversible and instantaneous compliance in the model, was associated with the network of all covalent bonds (with elastic properties) in cellulose microfibril, hemicellulose and pectin located in the primary cell wall microdomain, i.e., the oldest part of the cell wall. Such a microstructural assembly is located at the intermediated level between the secondary wall and middle lamella, and it is considered as the principal cell element able to counterbalance the intracellular turgor pressure from plasma membrane by engaging mainly the cellulose backbone, which the heat cannot degrade. As reviewed by Cosgrove [
31], cellulose microfibrils are deposited in the plane in the cell wall microdomain, thus it was expected to greatly influence the in-plane component of the volumetric elastic modulus. Two in-parallel interacting viscoelastic spring-dashpot arrays (Voigt elements,
J2, and
J3), describing the second and third components of the time-dependent reversible compliance in the model, were associated with the entangled network assemblies of cellulose microfibril, hemicelluloses, and pectin located in the primary cell wall and middle lamella, respectively. The entangled network assemblies, as represented by the three spring-dashpot arrays, were cumulatively considered as the secondary cell element able to counterbalance turgor pressure by engaging simultaneously covalent bonds (with elastic properties) and cross-links (with time-dependent viscoelastic properties) that the heat can only partly degrade. One
in-series independent dashpot (plastic component of the Maxwell element), describing the steady-state compliance regime in the model (namely
JN), was associated with the gel network among calcium, pectin and structural proteins that spreads from the middle lamella microdomain to that of the primary cell wall, and that the heat can rapidly and completely degrade. As reviewed by Cosgrove [
31], pectin assemblies probably exert dominant control of the out-of-plane component of volumetric elastic modulus, thus in this work, it was considered responsible for the viscoplastic (irreversible) flow occurring during through cell-to-cell separation, without decompartmentalization.
The overall compliance of cauliflower in the range of small-scale deformation was mathematically described using the following tailored Burger’s function:
J0 (kPa−1) is the instantaneous reversible compliance associated with the elastic stretching of the covalent bonds having the highest degree of freedom in the linear polymer fractions present in the cellulose microfibril network.
Ji (kPa−1) is the retarded reversible compliance of the ith-viscoelastic elements in the cellulose-hemicellulose-pectin network (they are associated with the stretching of covalent cross-links, the rate of which can be slow down by the simultaneous disentanglements/disassembling of non-covalent bonds interacting each other).
RTi (s) are the characteristic relaxation times governing the retarded compliance under viscoelastic regime.
JN (kPa−1) is the steady-state irreversible compliance. It is associated with the rupture of non-covalent bonds (calcium-pectin gel) in the middle lamella and among the structural proteins of the cell wall, both enabling a viscoplastic flow through partial cell separation and cell wall rupture.
All six replicates of the experimental creep curves were used simultaneously as an input in the non-linear regression of the proposed elastoplastic model (Equation (1)) accounting for an increasing number of the viscoelastic elements, and Durbin-Watson parameter was used as criterion to retain in the model the three fitting terms, namely J1, J2, and J3, with the lowest degree of correlation among model residues with zero mean.
The average value of all Burger’s model parameters was estimated under all investigated heating conditions and the lack-of-fit of the model was evaluated by calculating the square relative mean error
The average values of the Burger’s model parameters are reported in
Table 1.
E% resulted to be always less than 2, suggesting that the structure-function relationships hypothesis was adequate to satisfactorily describe the overall creep behavior of cauliflower in the range of small-scale deformation before and after heating.
The main advantage of tailoring the generalized Burgers’ function was to evaluate in an independent way the effect of the heat on the cauliflower tissue in reducing the effectiveness of the individual elastic, viscoelastic and plastic components of the polymeric assemblies located in the cell wall and middle lamella microdomains to counterbalance the residual turgor pressure under stresses equilibrium. The role of the individual elastic, viscoelastic and plastic elements of the polymer assemblies in the cell wall and middle lamella microdomains underlying the biophysics of thermal softening on a macroscopic scale as well as cell-to-cell separation, cell wall loosening and phytochemical decompartmentalization on a microscopic scale is pictured in
Scheme 3.
As reviewed by Jarvis et al. [
35], pectin is the only polymer present in a tricellular junction joining the parenchymatic cells, and the mechanical stress induced by cell turgor is distributed at tricellular junctions with two components: the first stress component acting at cell wall level on the plane of each cell-to-cell contact, and the second stress component acting at middle lamella level as radial stress separating the cells at the tricellular joining corners. In this work, phospholipidic membrane was assumed as the most heat-sensitive cell element that can be immediately degraded by the heat, thus causing partial loss in turgor pressure and its two stress components in the early step of heating and complete loss of turgor after prolonged heating. The second stress component of the turgor pressure surviving on heating was considered responsible of the cell separation mechanism causing the increase in creep compliance during the steady-state deformation regime under creep (
Figure 3). Cell separation on heating was associated with the degradation of pectin assemblies in the middle lamella microdomains. Otherwise, the first stress component of the turgor pressure that survives on heating was considered responsible of the increasing in creep compliance through partial rupture or loosening mechanisms of the cell wall and, therefore of the phytochemical decompartmentalization on a microscopic scale. Cell wall loosening or rupture on heating was associated with the degradation of the pectin assemblies in the cell wall microdomains, causing loss in the cell wall thickness or increasing of porosity, respectively.
Properties algebraically derived from the Burgers’ model parameters were proposed as descriptors of the overall creep compliance as well as at cell wall or middle lamella microdomains. The integrated compliance parameter (Ur expressed as s/kPa) was calculated as numerical integral from zero to the time at load removing of the creep compliance curve and used to represents the energy releasing rate under creep. An increase of the Ur parameter as induced by heat was treated as a quantitative measure of the cumulative softening of tissue microstructure across the two microdomains, i.e., cell wall and middle lamella. Since the compliance is an extensive property, the reversible compliance parameter, namely JR, was calculated as sum of the instantaneous J0 and retarded (J1, J2, and J3) compliances, and used to describe the cumulative changes as induced by heat in the three viscoelastic domains located in the primary and secondary cell wall and middle lamella. The ratios J0/Ur, ΣJi/Ur (with ΣJi calculated as sum of J, and J3) and JN/Ur were also calculated and used to compare the relative contributions from the elastic, viscoelastic and plastic components to the thermal softening.
The relative contributions from the elastic
J0/
Ur, viscoelastic Σ
Ji/
Ur and plastic
JN/
Ur cell components to the tissue microstructure compliance were compared in
Figure 4.
As it can be argued from figure, the three rheological parameters changes with the time of heating according to synchronous trends, suggesting that the two varieties of cauliflower are characterized by a tissue microstructure having the same microstructure hierarchy and polymer interactions at a cellular scale. Data also confirm that the relative contribution of cell elements to the overall creep compliance followed a decreasing order among viscoelastic, elastic and plastic supramolecular assemblies, either in the absence of the heat and after heating. As a consequence, the non-covalent network among cellulose, hemicellulose and pectin assemblies can be recognized as the most compliant and heat-sensitive cell element, irrespectively of the cauliflower variety and heating technology. In other words, the interconnections inside cellulose, hemicellulose and pectin assemblies represent the key scale level on which texture softening and tissue decompartmentalization are mainly depending on a microscopic scale.
Changes induced by heat in terms of the integrated compliance (
Ur) as well as of the reversible (
JR) and irreversible (
JN) components of the compliance are reported in
Figure 5.
Overall softening of tissue microstructure as represented cumulatively by
Ur progressed with the time of heating according to a sigmoidal kinetics, while an asynchronous trend was observed between
JR and
JN. However, a common feature was observed during cooking among the rheological parameters. An apparent breakpoint was detected during the early step of heating (close to 10 min), easier to detect under boiling than steaming and
sous-vide. The reversible compliance (
JR) increased considerably (both the elastic springs and viscoelastic elements in the model were lost partially and promptly) toward an asymptotic level, whereas the irreversible compliance (
JN) reached maximum level within 10 min of heating after which it decreased with the time of heating. Such a result was in agreement with literature. As reviewed by Ranganathan et al. [
1] and more recently by Liu et al. [
36], thermal softening in vegetables arises from two competing first-order mechanisms. The first mechanism was associated with chemical depolymerization of pectic substances in the middle lamella region via β-elimination mechanisms of their side chains, accounting for the loss of 85–97% of the original tissue firmness; the second mechanism was associated with the strengthening of the residual texture after prolonged heating through two independent firming mechanisms mediated by endogenous enzymes.
Under our experimental conditions, the synchronous increasing in
JR and
JN under creep after 10 min of heating was associated with two coupled relaxing mechanisms: (1) physical disruption of the bilayer phospholipidic membranes that cause partial loss in turgor, the surviving second stress component of which became less able to resist the external load, and (2) reorganization of viscoelastic and plastic elements in the middle lamella microdomain caused by water solubilization of calcium-pectic assemblies, thermal denaturation of structural proteins and chemical depolymerization via β-elimination of pectin side chains, all concurring locally to an increase of polymer mobility and to a decrease of cell-to-cell cohesiveness. Studies focused on carrots showed that many changes in pectin and loss in cellular turgor occur in early heating stages (namely within 3–10 min) when temperature reaches 100 °C and further changes are apparent after several minutes (30 min) when firmness is lost [
37].
Under our experimental conditions, the asymptotic increase of
JR occurring during the prolonged heating (from 10 to 40 min) was associated with the loosening (or partial rupture) of the viscoelastic elements extending from middle lamella microdomain (where the rate of the chemical depolymerization of pectin is slowing down by the enzymatic demethylesterification) to the primary and secondary cell wall microdomains, resulting in a decrease in cell wall porosity and thickness. Borowski et al. [
38] provided evidence that parenchymatic cell wall of broccoli boiled in water undergo loosening and dissolution as indicated by the highest content of total pectin, protopectin and water-soluble pectin fractions in boiling water, while steamed samples undergo partial rupture of cell wall (with only 1–2% of dry matter loss). Similar results and hypotheses were provided by Christiaens et al. [
39].
Under our experimental conditions, the decrease of
JN from 10 to 40 min was associated with a partial strengthening of pectin assemblies in the middle lamella microdomain, more likely due to the enzymatic demethylesterification of pectin (mediated by endogenous pectinmethylesterases and polygalacturonases) followed by polymer reorganization through pectin-calcium binding. It is widely documented in literature that the endogenous pectinmethylesterase may act (i) randomly in de-methylesterification reactions of pectin side chains, thus promoting the action of endogenous polygalacturonase that results in the loss of firmness and decrease in susceptibility of pectin to further chemical degradation, and (ii) linearly, enhancing the formation of calcium bridges between free carbonyl groups of demethylesterified pectin chains forming pectate-calcium complexes, thus causing partial strengthening in the middle lamella. Changes in
JN from 10 to 40 min of heating were more pronounced under steaming and
sous-vide than under boiling, irrespectively of the cauliflower variety. Such a result suggested that boiling treatments conducted under our conditions probably were not effective to activate the endogenous pectinmethylesterases. As reported by Kapusta-Ducth et al. [
15] in green and purple cauliflower, hydrothermal treatments cause consistent leakage of potassium and calcium in the hot water during the early phase of heating: this is another factor because the water boiling technology does not permit the consistent activation of pectinmethylesterases.
Under our experimental conditions, changes in
JR were more pronounced under boiling, whereas changes in
JN were more pronounced under steaming and
sous-vide. This indicates that boiling was the most effective heating treatment to enhance loosening or rupture in the cell wall microdomain during early heating as well as that steaming and
sous-vide provide the most of changes in the middle lamella microdomain. As reviewed by Baldwin [
40],
sous-vide cooking has shown to leave the plant cell wall mostly intact, and around 82–85 °C, it makes the vegetables tender by dissolving the cementing material that holds the cells together.
As far as the effect of heating on the two investigated varieties of cauliflower is concerned, although fresh samples showed no significant differences in terms of
Ur, the starting level of both
JR and
JN were in the
Cheddar lower than in the
Depurple. Furthermore, softening kinetics as described by
Ur,
JR, and
JN was faster and more pronounced in
Cheddar variety than in the
Depurple one and it progressed with a decreasing rate under boiling, steaming and
sous-vide. Such differences were in close agreement with texture softening determined on a macroscopic scale through the large-scale deformation experiments. Differences in the starting levels of
JR and
JN as well as in softening kinetics between the two varieties of cauliflower were tentatively explained by supposing that the degree of pectin methylesterification in the middle lamella microdomain was in
Cheddar cauliflower higher than in the
Depurple one. Such a hypothesis was according to the asynchronous contributions from viscoelastic and plastic cell elements as described by the model parameters J
R and
JN, respectively. In particular, untreated samples belonging to the
Depurple variety were characterized by higher level of reversible compliance (
JR). A higher number of methyl groups in the untreated samples will imply a greater proportion of hydrogen bonds and a higher creep compliance of both the viscoelastic (
JR) and plastic (
JN) cell wall elements. Additively, a higher number of methyl groups may accelerate the pectin depolymerization during heating, resulting in faster softening during the early step of heating (
Figure 5b,d) as well as in faster strengthening through calcium-pectin binding during prolonged heating (
Figure 5f). Our evidence-supported hypotheses concerning the contribution of the degree of pectin methylesterification in the mechanical properties as well as about the relationship between the softening rate and degree of methylation were in agreement with literature. As reviewed by Levesque-Tremblay et al. [
41] changes in degree of methylesterification result in changes of cell wall elasticity in meristematic tissues. Fraeye et al. [
42] provided evidence that high degree of methylesterification results in a more pronounced increase in the rate constant of chemical pectin depolymerization via β-elimination mechanisms rather than in the rate constant of enzymatic de-methylesterification. Fraeye et al. [
43] reported that pectin with low degree of methylesterification promotes cation binding of pectin. Constenla and Lozano [
44] showed that depolymerization of pectin in aqueous solutions causes an exponential reduction of the intrinsic viscosity (to one half after 40 min of heating at 80 °C) of the pectin polymer as a linear function of the degree of methylesterification.
Differences in softening kinetics (as described by Ur, JR, and JN) among the investigated heating conditions were explained by supposing that pectin degradation undergoes chemical and enzymatic reactions with different rate and extent as a function of the actual time-temperature conditions experienced by the cauliflower during heating. In particular, the enzymatic degrading mechanism was supposed to be activated selectively according to the rate of increasing in temperature, reflecting the specific heat-transfer efficiency of the cooking methods. Steam condenses on the surface of the products and a large amount of latent heat transfers to the plant tissue with an intermediate rate between boiling and sous-vide.
It is largely accepted that, the optimal range of temperature for endogenous pectinmethylesterases typically is 50–80 °C, after which the enzymes are progressively inactivated in vegetables. Li Ni et al. [
45] reported that pectinmethylesterases can be activated by heat activity in broccoli between 50 and 55 °C as well as from 50 to 70 °C in choy midribs; however, thermal stability of the pectinmethylesterases is lost during heating by following the first-order kinetics in the range of 70–95 °C. Pectinmethylesterases are completely inactivated in broccoli after 5 min treatment at 80 °C [
46].
Under our experimental conditions, the time of heating experienced by the samples in the temperature range potentially activating the endogenous pectinmethylesterases followed a decreasing order under boiling, steaming and
sous-vide. For the sake of example,
Figure 6 shows the time-temperature profiles of 25 min boiling, steaming and
sous-vide treatments for
Cheddar cauliflower. As can be inferred from data, samples experienced the increase in temperature from 40 to 80 °C in about 1.83, 2.67, and 5.5 min under boiling, steaming and
sous-vide treatments, respectively. Furthermore, the time experienced at temperature higher than 80 °C was about 22.5, 17.6, and 13.5 min, respectively.
Due to the relatively short time experienced to reach the temperature potentially activating the endogenous pectinmethylesterases as well as the relatively long time experienced under temperatures inactivating the enzymes, the extent of pectin de-methylesterification during boiling treatment was supposedly limited versus chemical depolymerization via β-elimination reactions of pectin side chains, thus enhancing cell wall loosening (or rupture) with respect to cell separation when compared against steaming and
sous-vide treatments. Our hypotheses were in agreement with literature. Constenla and Lozano [
44] showed that relatively high temperatures (>80 °C) accelerate the first-order β-eliminative depolymerization of pectin, while pectin de-methylesterification mediated by the pectinmethylesterases follow a second-order kinetics in the range of temperature 50–80 °C with the constant rate increasing exponentially with temperature.
3.3. Profiling Extractability of Sterol, Tocopherol, and Water
Figure 7 compares the total amount of the sterols and tocopherols as well as of the water extracted before and after cooking (error bars represent the standard deviation).
Data related to the sterols and tocopherols were expressed on dry basis, while those referring to the water were expressed as percentage. The water extracted after
sous-vide treatments was associated with the release of the intercellular fraction of water bound in the middle lamella microdomain due to partial disassembly of the pectin-calcium hydrogel. Otherwise, the increasing in the water extracted after boiling and steaming treatments was associated with the water uptake from the heating medium (hot water and condensed steam, respectively) that was absorbed through hydrogen bonds by the cellulose, hemicellulose. and pectin assemblies in the cell wall microdomain as a function of the extent of degradation of their non-covalent interaction. As it can be argued from
Figure 7, the untreated samples belonging to the two varieties of cauliflower were characterized by considerable differences in terms of sterols and tocopherols, with less remarkable but statistically significant differences in terms of the intracellular water. In particular, the concentration of the extractable sterols and tocopherols as expressed on dry basis were 1422 ± 28.3 and 37.8 ± 13.3 mg/kg as well as 745.2 ± 12.5 and 28.5 ± 4.1 mg/kg in
Depurple and
Cheddar cauliflower, respectively; while the extracted water was 92.3% ± 0.1%, and 92.8% ± 0.1% in
Depurple and
Cheddar, respectively. The highest percentage of water was in agreement with our hypothesis of a higher degree of methylesterification in fresh
Cheddar with respect to
Depurple cauliflower: methyl groups are able to hold water molecules via hydrogen bonds. Comparing our results with literature, we found lower levels of sterols in raw cauliflower. In white cauliflower, sterols accounted for 276 mg/kg (wet basis) or 3186 mg/kg (dry basis) [
47], 274–411 mg/kg
w/
w or 4092–5274 mg/kg (dry basis) [
23] and 40 mg/100 g of edible portion [
48]. The lower values under our experimental conditions could be due to different extraction method applied: we performed only alkaline saponification of the starting sample, while the authors also performed the acid hydrolysis that is able to release the steryl glycoside sterols. Among vegetables, the highest levels of sterols, e.g., >300 mg/kg (wet basis), were found in broccoli, brussels sprouts, cauliflower, and dill [
23]. These higher values were supposed to be due to the higher content of meristematic tissues rich in cell membrane, especially those constituting cauliflower florets [
23].
Concerning boiling as the heating technology, the total amount of the extracted sterols, tocopherols as well as of the extracted water increased considerably with the time of heating. After 10 min of heating, the total amount of extracted sterols was on average 1.87 and 1.57 greater than the initial level in the
Depurple and
Cheddar cultivar, respectively, while the total amount of tocopherols was on average about 2.3 and 4.62 times greater than the initial level, respectively. After 25 min of heating, the sterols extracted from the two cultivars were about 2.40 and 5.13 times greater than the initial level, while the tocopherols were about 3.55 and 6.43 times, respectively. However, boiling allowed cauliflower tissues to absorb considerable amount of water from heating medium. The water extracted from 10 min boiled samples resulted on average 3.2% and 3.3% greater than the initial level in
Depurple and
Cheddar cauliflower, respectively. After 40 min of heating, the extracted water was on average 4.1% and 4.2% greater than the initial level in
Depurple and
Cheddar cauliflower, respectively. Such an increased water holding capacity was supposed to be the result of pectin degradation induced by the heat as well as of the changes in the interaction among cellulose, hemicellulose and pectin assemblies. As reviewed by Levesque-Tremblay et al. [
41], pectin softening is permissive of hydration. Changes in water holding capacity assume a key meaning also under a nutritional perspective. Referred as wet basis, the level of sterols extracted after 25 min of boiling was on average 1.24 and 2.44 times greater than the initial one for
Depurple and
Cheddar cauliflower, respectively. Otherwise, referring on wet basis the tocopherol concentration after 25 min of boiling, the actual increment was on average 1.21 and 3.05 times greater than the initial level for
Depurple and
Cheddar cauliflower, respectively.
Literature on sterol refers only to white cauliflower and boiling cooking. Kaloustian et al. [
47] claimed that 30 min of boiling increased the level of the free sterols in eight plant products, including cauliflower, if dry matter was considered. They found a double increment of extractable sterols (from 3186 to 6250 mg/kg expressed in dry basis) in boiling fresh cauliflower. The authors also concluded that cooked vegetables might give better protection against cardiovascular diseases due to higher sterol levels. Normén et al. [
48] found in boiled white cauliflower a decreasing level of extractable sterols with no significant differences with respect to other investigated vegetables (in this work the time of boiling was not specified). To the best of our knowledge, no literature is available on sterol extractability with respect to cooking technologies different from boiling (e.g., steaming and sous-vide).
Concerning steaming as heating technology, the total amount of the extracted sterols decreased at each time of heating in the
Depurple samples (
Figure 7a, differences were significant with
p < 0.05), reaching after 40 min on average about 0.50 times the initial level. Under our experimental conditions, the total amount of sterols increased with the time of heating in
Cheddar cauliflower, reaching after 40 min on average 2.32 times greater than the initial level (differences were significant with
p < 0.05). Conversely, the total amount of tocopherols extracted after 10 min of heating from was on average 1.70 and 2.97 times the initial level (
Figure 7b,e) in the
Depurple and
Cheddar samples, respectively. After 25 min of heating, the total amount of extracted tocopherols was on average 1.36 and 3.49 times the initial level in the
Depurple and
Cheddar samples, respectively. After 40 min of steaming, the total amount of extracted tocopherols was 1.50 and 3.20 times the initial level in the
Depurple and
Cheddar samples, respectively. Concerning the total amount of the water extracted after 10 min of steaming resulted on average of about 0.2% lower and 1.7% greater than the initial level in the
Depurple and
Cheddar samples, respectively. The pronounced absorption of water was in agreement with our hypothesis of a higher degree of pectin methylesterification in
Cheddar cauliflower. However, the high variability observed in the percentage of the water extracted at 10 min of steaming (
Figure 7c,f) suggested that this cooking technology resulted in non-uniform heating effects in the early steps of cooking in both the cauliflower varieties. An additional time of steaming, i.e., from 25 to 40 min, permitted a less considerable increase of extracted water, i.e., on average of about 0.5% in the
Depurple and 0.7% in
Cheddar samples, resulting in reduced variability. As reviewed by Xiao et al. [
49], although steaming causes weight loss less pronounced with respect to boiling, reducing leaching of nutrients in the condensed medium, and the formation of a dried layer on product surface due to evaporation of water, authors concluded that steaming results in non-uniform heating effects and needs longer treatment time than hot water, due to the lower heat transfer in steam than in hot water.
Concerning sous-vide as heating technology, the total amount of the extracted sterols from Depurple samples decreased considerably within 10 min of heating, then it moderately increased after 25 and 40 min of heating (p < 0.05). However, the sterols extracted after 40 min of heating were 0.9 times the initial level (untreated samples). Otherwise, the sterols extracted from Cheddar samples increased considerably at each time of heating: After 40 min it resulted on average 2.8 times greater than the initial level. The total amount of the extracted tocopherols after 40 min of heating increased moderately in Depurple samples and considerably in the Depurple samples: the final level was 1.4 and 3.0 times greater than the initial one (p < 0.05), respectively. The total amount of water extracted from Depurple samples heated under sous-vide undergo changes close to that observed in the same variety as heated under steaming. However, the amount of water extracted from Cheddar samples was relatively lower after each time of heating, with a significant increase after 10 min (p < 0.05).
Several studies suggested that considerable differences in compounds extractability among vegetables can be associated with the morphological structure and tissue thickness [
4].
In this study, the negative correlation of sterols extractability with time of steaming and
sous-vide in
Depurple and the positive correlation in
Cheddar cauliflower were explained supposing variety-dependent differences in pectin methylesterification and microstructure at a cellular scale. As reviewed by Liu et al. [
36] the effects of processing on pectin structure are highly dependent on the processing conditions: pectin with low degree of methylesterification caused lower bioaccessibility of both lipophilic and hydrophilic bioactive compounds as compared to pectin with high degree of methylesterification, which may be attributed to higher pectin binding capacities. Localized stiffness in cell wall microdomains and in cell membrane were also proposed as two additional variety-related factors affecting functional properties of the cell wall, in the sense of its effectiveness in decompartmentalize sterols as affected by heat: local heterogeneity affected pectin degradation unevenly throughout primary cell wall microdomain thus explaining the apparent synchrony in terms of compliances (as described by
Ur,
JR, and
JN in
Figure 5) and the apparent asynchrony in terms of extractability of sterols and tocopherols under steaming and
sous-vide (as reported in
Figure 7a,b,d,e). A non-homogeneous distribution of linear pectin of various methylesterification degrees and patterns is documented in literature as a key factor contributing to spatial differences in the elasticity, hydration, porosity, and adhesion properties, either at a cell wall scale and tissue scale. As reviewed by Levesque-Tremblay et al. [
41], as high as 80% of homogalacturonan (linear) pectin in growing cells is methyl esterified before its secretion from Golgi apparatus to cell wall where it redistributes and concentrates locally after de-methylesterification mediated by endogenous pectinmethylesterases. De-methylesterified homogalacturonan may encounter two general fates: (1) the formation of a stable structure with other homogalacturonan molecules (causing local strengthening of the cell wall), or (2) degradation by polygalacturonases (causing local loosening of the cell wall). As reviewed by Turner and Kumar [
25], sterols may play an indirect role in cellulose biosynthesis enabling the processes of acylation of targeted membrane proteins and of membrane partitioning into sterol-rich microdomains in the region of cellulose synthesis. As consequence, local heterogeneity in the cell wall and cell membrane microstructure may be expected as plant variety-specific properties, thus providing a possible explanation because sterol extractability decreased in
Depurple and increased in
Cheddar when they were treated under lower heat transfer efficiency conditions, i.e., steaming and
sous-vide.
The extractability profiling was analyzed also with respect to the main differences observed in terms of the individual active compounds. The level of individual sterols and tocopherols are reported in
Table 2 (mg/kg of dry basis) and
Table 3 (mg/kg of wet basis) together with their 95% confidence limits.
As it can be argued from data, in all samples (untreated and cooked) β-sitosterol was the major sterol followed by campesterol and stigmasterol.
Depurple cauliflower was 2-fold richer in campesterol, stigmasterol and β-sitosterol than
Cheddar. Our hypothesis of a lower degree of the methylesterification and higher compliance in fresh
Depurple cauliflower was corroborated by the corresponding higher concentration of sterols, mainly β-sitosterol and campesterol (
Table 2 and
Table 3), in the cell membrane. Da Silveira et al. [
50] reported that high sterol content (with a decreasing order between β-sitosterol, campesterol and stigmasterol) is related to an increment of the cell membrane thickness and of turgor. Greve et al. [
37] showed that a higher level of turgor in fresh carrots causes more rapid loss in firmness during the early boiling (1 min).
Expressing data on fresh vegetables, the major positive effect on phytosterols levels in violet cauliflower was recorded after boiling (10 and 25 min with no statistical difference between minutes) and the major negative effect after steaming at 40 min and sous-vide cooking at 10 min.
3.4. Interplay between Phytochemical Extractability, Cell Separation and Cell Wall Rupture
The profiles of the total sterols and tocopherols extracted after cooking were analyzed as a function of the impact of heat on tissue microstructure as described by the rheological parameters determined in the range of both the large and small scale of deformation.
Concerning the range of large-scale deformation (macroscopic scale), the loss of the stiffness in
Cheddar cauliflower (
Figure 2) after 25 min of
sous-vide, steaming and boiling, were coherent with the increasing in total concentration of sterols and tocopherols extracted after cooking. The amount of the individual sterols and tocopherols are reported in
Table 2 and
Table 3. Sterols were 1269.3 ± 203.2, 1908.5 ± 172.8, and 3822.0 ± 57.8 mg/kg, respectively, while tocopherols were 83.3 ± 10.2, 99.7 ± 16.5, and 183.4 ± 24.1 mg/kg, respectively. Results support the idea that the ease to extract the two classes of bioactive macromolecules is strictly related to thermal softening on a macroscopic scale. However, for extractability to increase, hierarchical disassembling of tissue microstructure is required on a microscopic scale, through different potential mechanisms which include cell membrane disruption and releasing of molecules entrapped in the phospholipid by-layer, pectin degradation extending from middle lamella (causing cell separation) to cell wall loosening (causing increasing of porosity or loss in the cell wall thickness). Prevalence of cell wall loosening to cell separation mechanisms must be considered as the main cause of the increase of macromolecules extractability and of their bioaccessibility during consumption.
With the aim to investigate the impact of cooking in terms of the tissue decompartmentalization and its relation on the extractability of sterols and tocopherols, two microstructure-related parameters were defined using the rheological parameters determined in the range of small scale of deformation, namely the “Elastic Recovery Ability, ERA” and the “Elastic-to-Viscoplastic Ratio, EPR”.
The
ERA parameter was calculated as in the following:
ERA was used as a descriptor of the recoverable elasticity at a cell wall scale, thus positively related to the ability to bear turgor pressure, and negatively to the phytochemicals extractability. ERA may vary from 100 (complete recovery of microstructure elasticity) to 0 (complete loss of microstructure elasticity). Decreasing in the ERA parameter as induced by heat were treated as indirect measure of the residual cell wall integrity due to the cumulative damage in terms of cell membrane disassembling (loss of turgor) and irreversible pectin degradation in both the cell wall and middle lamella microdomains.
The
EPR parameter was calculated as in the following:
EPR was used as a descriptor of the prevalence of the disassembling mechanisms causing cell wall loosening to those causing cells separation without cell rupture, thus positively related to the easy to extract sterols and tocopherols.
Values of
ERA and
EPR are reported in
Table 1. As can be argued from data, the two varieties showed the same initial ability to prevent cell loosening and cell separation mechanisms as indicated by the level of
EPR in the untreated samples belonging to the two varieties (difference was not significant,
p > 0.05). However, they were characterized by different type of interaction among the cell wall polymers, and therefore different ability to counterbalance turgor pressure and to resist pectin degradation during boiling, steaming, and
sous-vide. The untreated
Depurple cauliflower showed the highest level of
ERA. Moreover,
ERA decreased continuously over cooking with an increasing order under
sous-vide, steaming and boiling conditions. The
ERA decreasing rate was in
Cheddar cauliflower greater than in the
Depurple one. Such results suggested that fresh
Depurple cauliflower was characterized by strengthen cell wall with an ability to bear turgor pressure and resist pectin degradation under heating higher than in the
Cheddar cauliflower. Changes in polymer interaction during heating affected differently the two cultivars to resist cell loosening and cell separation. The level of
EPR registered under boiling and steaming conditions reached a minimum value at 10 min of heating after which it increased in
Cheddar cauliflower with a rate greater than in the
Depurple one, thus highlighting a variety-specific ability to shift mechanisms causing cell separation to cell wall loosening.
To obtain insight on the interplay relating cooking technology, heating time, tissue softening, cell wall loosening and phytochemical extractability, the parameters
ERA,
EPR, and
Ur together with the total amount of sterols (
TotSte) and of tocopherols (
TotToc) as well as the percentage of water extracted (
W%) were treated as active variables in a principal component analysis (PCA). Campesterol, stigmasterol, β-sitosterol as well as γ-tocopherol and α-tocopherol together with the
EtaN and
E0 parameters were treated as supplementary variables aiming to full characterize the samples under cooking. Results from PCA are reported in the
Figure 8, in which the samples belonging to
Depurple variety are highlighted in blue and those belonging to
Cheddar in red.
The score loadings corresponding to the samples treated under the same cooking technology were also connected with consecutive arrows to trace the pattern of microstructural changes during heating (from 0 to 40 min). As can be argued from figure, the pattern of changes was similar under the same cooking technology, but satisfactorily discriminated the samples belonging to Depurple variety from those belonging to the Cheddar one. The first three principal components explained more than 93% of the experimental variance (73.44%, 14.76%, and 11.8%, respectively). The ERA parameter was positively correlated to E0 and opposite to EPR and Ur. EPR was also opposite to Ur and EtaN. Such relationships corroborated the idea that the covalent bonds (as indicated by E0) are positively linked to both the turgor pressure and cell wall integrity (as indicated by ERA), and that cell separation (as indicated by low levels of EPR) is the main cause of softening (as expressed in terms of Ur) resulting in a decrease of non-covalent bonds (as indicated by low levels in EtaN). According to the magnitude and signs of the factor coordinates of the two microstructural-related parameters ERA and EPR, the first two principal components (PC 1 and PC 2) were meaningfully labeled as to “Cell Wall Integrity” and “Cell Separation”, respectively. Magnitude and signs of the factor coordinates also indicated that TotToc, TotSte, and W% were positively correlated to EPR and Ur as well as that they were negatively correlated to ERA. Such results provided compelling evidence that the extractability of sterols and tocopherols was in agreement with the loss of cell wall integrity (as described by ERA) and that it was in Cheddar cauliflower higher than in Depurple. The extractability of the phytochemicals increases with the time of heating to such an extent depending on the ability of the cooking technology to enhance cell wall loosening to a greater extent with respect to the viscous flow in the middle lamella through cell separation, thus achieving low levels of ERA and high levels of EPR.
The time-dependent pattern of changes in terms of loss of cell wall integrity rather than cell separation can be advantageously analyzed by the range of variation of the two principal components with respect to the time of cooking.
PC 1 assumed positive sign throughout the entire period of heating under steaming and sous-vide, while it decreased from positive to negative values under boiling.
Additively, the range of variation of PC 1 was always lower in Depurple cauliflower than that characterizing Cheddar, most likely due to the nonhomogeneous distribution of pectin degradation at a local scale in under low efficiency in both heat transfer conditions. The range of variation of PC1 followed the decreasing order under boiling, steaming and sous-vide. The opposite can be observed for the PC 2. This reflects the idea that boiling was the most effective cooking method to enhance the mechanisms causing tissue decompartmentalization through cell wall loosening (a, b) with respect to those causing cell separation (c, d) having no impact on the phytochemical extractability. Sous-vide showed the lowest impact on cell membrane and cell wall integrity, but the highest in terms of cell separation. Steaming showed an intermediate behavior.
Table 4 lists the multiple regression models able to predict the concentration of sterols, tocopherols (on dry basis) that can be extracted from cauliflower after cooking by using
ERA and
EPR as input predictors. The regression coefficients are reported together with their standard errors.