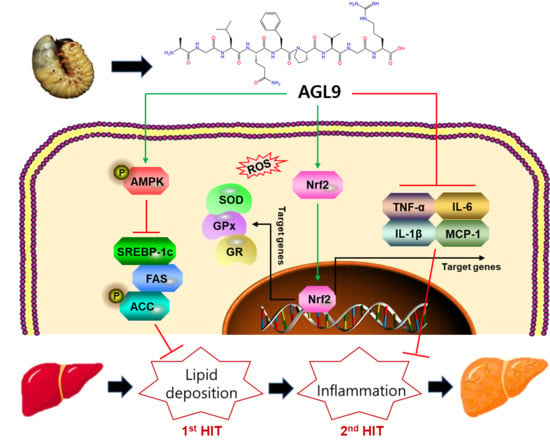
AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture, Differentiation, and Staining
2.3. Dosage Information/Regimen
2.4. Histological Evaluation
2.5. Immunohistochemistry
2.6. Biochemical Analysis
2.7. Evaluation of the Transcript-Level Expression of Target Genes Using Quantitative Real-Time PCR (qPCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. The A. dichotoma Larva Enzymatic Hydrolysate Improves Lipid Deposition in 3T3-L1 Cells
3.2. Effect of AGL9 on Liver Injury in NAFLD Mice
3.3. Effect of AGL9 on Lipid Metabolism in NAFLD Mice
3.4. Effect of AGL9 on AMPK Signaling in NAFLD Mice
3.5. Effects of AGL9 on Oxidative Stress and Inflammation in the Liver of NAFLD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, N.; Kimura, T.; Fujimori, N.; Nagaya, T.; Komatsu, M.; Tanaka, E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 2019, 25, 163–177. [Google Scholar] [CrossRef]
- Overi, D.; Carpino, G.; Franchitto, A.; Onori, P.; Gaudio, E. Hepatocyte injury and hepatic stem cell niche in the progression of non-alcoholic steatohepatitis. Cells 2020, 9, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Temple, J.L.; Cordero, P.; Li, J.; Nguyen, V.; Oben, J.A. A guide to non-alcoholic fatty liver disease in childhood and adolescence. Int. J. Mol. Sci. 2016, 17, 947. [Google Scholar] [CrossRef]
- Geh, D.; Manas, D.M.; Reeves, H.L. Hepatocellular carcinoma in non-alcoholic fatty liver disease—A review of an emerging challenge facing clinicians. Hepatobiliary Surg. Nutr. 2021, 10, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Duan, R.; Zhong, H.; Liang, T.; Guo, L. The crosstalk between fat homeostasis and liver regional immunity in NAFLD. J. Immunol. Res. 2019, 2019, 3954890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolasevic, I.; Milic, S.; Wensveen, T.T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Orlic, L. Nonalcoholic fatty liver disease-A multisystem disease? World J. Gastroenterol. 2016, 22, 9488–9505. [Google Scholar] [CrossRef]
- He, Y.; Jiang, J.; He, B.; Shi, Z. Chemical activators of the Nrf2 signaling pathway in nonalcoholic fatty liver disease. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [Green Version]
- Houttu, V.; Boulund, U.; Grefhorst, A.; Soeters, M.R.; Pinto-Sietsma, S.J.; Nieuwdorp, M.; Holleboom, A.G. The role of the gut microbiome and exercise in non-alcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Bækdal, T.A.; Thomsen, M.; Kupčová, V.; Hansen, C.W.; Anderson, T.W. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J. Clin. Pharmacol. 2018, 58, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Yu, X.Y.; Ma, X.F.; Lin, W.J.; Hao, M.; Kuang, H.Y. Exenatide delays the progression of nonalcoholic fatty liver disease in C57BL/6 mice, which may involve inhibition of the NLRP3 inflammasome through the mitophagy pathway. Gastroenterol. Res. Pract. 2018, 2018, 1864307. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Knop, F.K.; Vilsbøll, T. Effects of lixisenatide on elevated liver transaminases: Systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open 2014, 4, e005325. [Google Scholar] [CrossRef]
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Jung, C. Insects used as food and feed: Isn’t that what we all need? Foods 2020, 9, 502. [Google Scholar] [CrossRef]
- Bae, S.M.; Fan, M.; Choi, Y.J.; Tang, Y.; Jeong, G.; Myung, K.; Kim, E.K. Exploring the Role of a Novel Peptide from Allomyrina dichotoma Larvae in Ameliorating Lipid Metabolism in Obesity. Int. J. Mol. Sci. 2020, 21, 8537. [Google Scholar] [CrossRef]
- Fan, M.; Choi, Y.J.; Tang, Y.; Bae, S.M.; Yang, H.P.; Kim, E.K. Efficacy and mechanism of polymerized anthocyanin from grape-skin extract on high-fat-diet-induced nonalcoholic fatty liver disease. Nutrients 2019, 11, 2586. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.S.; Chen, W.C.; Kuo, T.C.; Ho, C.T.; Kuo, C.H.; Tseng, Y.J.; Sheen, L.Y. Mass-spectrometry-based serum metabolomics of a C57BL/6J mouse model of high-fat-diet-induced non-alcoholic fatty liver disease development. J. Agric. Food Chem. 2015, 63, 7873–7884. [Google Scholar] [CrossRef]
- Campos-Murguía, A.; Ruiz-Margáin, A.; González-Regueiro, J.A.; Macías-Rodríguez, R.U. Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 5919–5943. [Google Scholar] [CrossRef]
- Wang, H.; Mehal, W.; Nagy, L.E.; Rotman, Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell. Mol. Immunol. 2021, 18, 73–91. [Google Scholar] [CrossRef]
- Treem, W.R.; Palmer, M.; Lonjon-Domanec, I.; Seekins, D.; Dimick-Santos, L.; Avigan, M.I.; Chalasani, N. Consensus Guidelines: Best practices for detection, assessment and management of suspected acute drug-induced liver injury during clinical trials in adults with chronic viral hepatitis and adults with cirrhosis secondary to hepatitis B, C and nonalcoholic steatohepatitis. Drug Saf. 2021, 44, 133–165. [Google Scholar]
- Ullah, R.; Rauf, N.; Nabi, G.; Ullah, H.; Shen, Y.; Zhou, Y.D.; Fu, J. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: Recent updates. Int. J. Biol. Sci. 2019, 15, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.X.; Wei, J.D.; Mu, J.K.; Liu, X.; Li, F.J.; Li, Y.Q.; Yu, J. Mitochondrial metabolomic profiling for elucidating the alleviating potential of Polygonatum kingianum against high-fat diet-induced nonalcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 6404–6415. [Google Scholar] [CrossRef] [PubMed]
- Febriza, A.; Ridwan, R.; As’ad, S.; Kasim, V.N.; Idrus, H.H. Adiponectin and Its Role in Inflammatory Process of Obesity. Mol. Cell. Biomed. Sci. 2019, 3, 60–66. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Chen, X.; Li, Y.; Ke, Y. SeP is elevated in NAFLD and participates in NAFLD pathogenesis through AMPK/ACC pathway. J. Cell. Physiol. 2021, 236, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic effect of shikimic acid via inhibition of MID1IP1 and phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef] [Green Version]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Nakamuta, M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.J.; Kwon, S.W.; Jung, B.H.; Oh, S.H.; Lee, B.H. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J. Lipid Res. 2011, 52, 1617–1625. [Google Scholar] [CrossRef] [Green Version]
- Besse-Patin, A.; Estall, J.L. An intimate relationship between ROS and insulin signalling: Implications for antioxidant treatment of fatty liver disease. Int. J. Cell Biol. 2014, 2014, 519153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Sharma, A.; Duseja, A.; Das, A.; Dhiman, R.K.; Chawla, Y.K.; Bhansali, A. Patients with nonalcoholic fatty liver disease (NAFLD) have higher oxidative stress in comparison to chronic viral hepatitis. J. Clin. Exp. Hepatol. 2013, 3, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem.-Biol. Interact. 2008, 172, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Shen, P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Feng, H.; Cheng, J.; Li, Z.; Jin, M.; Zhao, L.; Liu, G. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J. Cell. Mol. Med. 2020, 24, 5097–5108. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.L.; Yu, B.; Li, Z.; Jiang, W.X.; Jiang, J.D.; Kong, W.J. Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway. Phytother. Res. 2016, 30, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C.; Zhang, L.; Zhao, Y.; Duan, C.; Zhang, X.; Li, S. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 2019, 103, 5843–5850. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. BBA-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]






| Gene Name | Sequence | |
|---|---|---|
| Atgl | Forward | 5′-GAC CTG ATG ACC ACC CTT TCC-′3 |
| Reverse | 5′-TGC TAC CCG TCT GCT CTT TCA-′3 | |
| Sod | Forward | 5′-CAA TGG TGG GGG ACA TAT TA-′3 |
| Reverse | 5′-TTG ATA GCC TCC AGC AAC TC-′3 | |
| Gpx | Forward | 5′-ACA TTC CCA GTC ATT CTA CC-′3 |
| Reverse | 5′-TTC AAG CAG GCA GAT ACG-′3 | |
| Nr3c1 | Forward | 5′-CGG CGA TCT CCA CAG CAA TG-′3 |
| Reverse | 5′-ACC GCT CCA CAC ATC CTG ATT G-′3 | |
| Nrf2 | Forward | 5′-AGC ACA TCC AGA CAG ACA CCA GT-′3 |
| Reverse | 5′-TTC AGC GTG GCT GGG GAT AT-′3 | |
| Tnfa | Forward | 5′-AAG CCT GTA GCC CAC GTC GT-′3 |
| Reverse | 5′-GGC ACC ACT AGT TGG TTG TC-′3 | |
| Il1b | Forward | 5′-AAC CAA GCA ACG AVA AAA TA-′3 |
| Reverse | 5′-AGG TGC TGA TGT ACC AGT TG-′3 | |
| Il6 | Forward | 5′-CCG GAG AGG AGA CTT CAC AG-′3 |
| Reverse | 5′-GGA AAT TGG GGT AGG AAG GA-′3 | |
| Mcp1 | Forward | 5′-TGA TCC CAA TGA GTA GGC TGG AG-′3 |
| Reverse | 5′-ATG TCT GGA CCC ATT CCT TCT TG-′3 | |
| Gapdh | Forward | 5′-GCA CAG TCA AGG CCG AGA AT-′3 |
| Reverse | 5′-GCC TTC TCC ATG GTG GTG AA-′3 |
| Parameter | CON | NAFLD | AGL9 | Orlistat | |
|---|---|---|---|---|---|
| Serum | TG (mg/dL) | 108.33 ± 2.52 c | 232.60 ± 3.42 a | 138.76 ± 6.25 b | 117.92 ± 4.72 c |
| TC (mg/dL) | 96.06 ± 4.72 b | 119.65 ± 6.03 a | 120.46 ± 4.32 a | 119.26 ± 2.80 a | |
| HDL (mg/dL) | 79.81 ± 4.79 a | 47.35 ± 6.03 b | 77.81 ± 1.23 a | 75.05 ± 2.80 a | |
| LDL/VLDL (mg/dL) | 16.25 ± 2.64 c | 72.30 ± 2.20 a | 42.65 ± 5.57 b | 44.21 ± 4.39 b | |
| Adiponectin (μg/mL) | 53.45 ± 4.46 b | 84.19 ± 5.52 a | 66.71 ± 4.26 b | 67.43 ± 5.11 b | |
| Leptin (ng/mL) | 4.80 ± 1.68 c | 45.40 ± 3.14 a | 22.08 ± 2.74 b | 20.08 ± 2.67 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.; Choi, Y.-J.; Tang, Y.; Kim, J.H.; Kim, B.-g.; Lee, B.; Bae, S.M.; Kim, E.-K. AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling. Foods 2021, 10, 1973. https://doi.org/10.3390/foods10091973
Fan M, Choi Y-J, Tang Y, Kim JH, Kim B-g, Lee B, Bae SM, Kim E-K. AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling. Foods. 2021; 10(9):1973. https://doi.org/10.3390/foods10091973
Chicago/Turabian StyleFan, Meiqi, Young-Jin Choi, Yujiao Tang, Ji Hye Kim, Byung-gyu Kim, Bokyung Lee, Sung Mun Bae, and Eun-Kyung Kim. 2021. "AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling" Foods 10, no. 9: 1973. https://doi.org/10.3390/foods10091973
APA StyleFan, M., Choi, Y. -J., Tang, Y., Kim, J. H., Kim, B. -g., Lee, B., Bae, S. M., & Kim, E. -K. (2021). AGL9: A Novel Hepatoprotective Peptide from the Larvae of Edible Insects Alleviates Obesity-Induced Hepatic Inflammation by Regulating AMPK/Nrf2 Signaling. Foods, 10(9), 1973. https://doi.org/10.3390/foods10091973