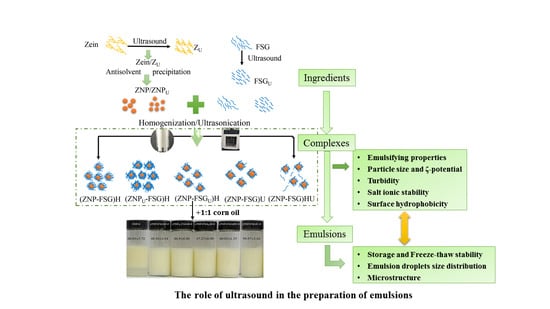
The Role of Ultrasound in the Preparation of Zein Nanoparticles/Flaxseed Gum Complexes for the Stabilization of Pickering Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Pretreatment of Samples
2.2.1. Preparation Individual Solution
2.2.2. Preparation of Zein Nanoparticles (ZNP)
2.2.3. Ultrasound Treatment
2.3. Preparation of Complex Particles
2.4. Preparation of Pickering Emulsions
2.5. Characterization of Complexes
2.5.1. Particle Size and Zeta (ζ)-Potential of Complexes
2.5.2. Turbidity Measurement
2.5.3. Salt Ionic Stability Measurement
2.5.4. Surface Hydrophobicity (H0)
2.5.5. Determination of Emulsifying Properties
2.6. Determination of Stability
2.6.1. Storage Stability of Pickering Emulsions
2.6.2. Freeze-Thaw Stability of Pickering Emulsions
2.7. Droplets Size of Pickering Emulsions
2.8. Microstructure Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Ultrasound Process on the Preparation of ZNP-FSG Complexes
3.1.1. Emulsifying Properties
3.1.2. Turbidity Changes
3.1.3. Salt Ionic Stability
3.1.4. Surface Hydrophobicity
3.1.5. Particle Size
3.1.6. ζ-Potential
3.2. Effects of Ultrasound Process on the Emulsion of ZNP-FSG Complexes
3.2.1. Storage Stability of Emulsions
3.2.2. Freeze-Thaw Stability of Emulsions
3.2.3. Emulsion Droplets Size Distribution
3.2.4. Microstructure of Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.Q.; Huang, Q.R. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.-G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.-M.; Feng, X.-Y.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Wei, Z.-J.; Wang, C.-Y.; Zou, S.-W.; Liu, H.; Tong, Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. [Google Scholar] [CrossRef]
- Ning, F.-J.; Ge, Z.-Z.; Qiu, L.; Wang, X.-Q.; Luo, L.-P.; Xiong, H.; Huang, Q.-R. Double-induced se-enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade pickering emulsions. Food Hydrocoll. 2020, 99, 105308. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.S.; Li, J.; Wei, X.L.; Li, B. Improving the emulsifying property of gliadin nanoparticles as stabilizer of Pickering emulsions: Modification with sodium carboxymethyl cellulose. Food Hydrocoll. 2020, 107, 105936. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.-L.; Liu, J.; Zhu, Y.; Zhang, X.-Y.; Jiang, L.-Z.; Qi, B.-K.; Zhang, X.-N.; Wang, Z.-J.; Teng, F. Soy Protein Isolate-Phosphatidylcholine Nanoemulsions Prepared Using High-Pressure Homogenization. Nanomaterials 2018, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.-Y.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- Zhang, K.-M.; Mao, Z.-J.; Huang, Y.-C.; Xu, Y.; Huang, C.-D.; Guo, Y.; Ren, X.-e.; Liu, C.-Y. Ultrasonic assisted water-in-oil emulsions encapsulating macro-molecular polysaccharide chitosan: Influence of molecular properties, emulsion viscosity and their stability. Ultrason. Sonochem. 2020, 64, 105018. [Google Scholar] [CrossRef]
- Albano, K.M.; Nicoletti, V.R. Ultrasound impact on whey protein concentrate-pectin complexes and in the O/W emulsions with low oil soybean content stabilization. Ultrason. Sonochem. 2018, 41, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.-Y.; Pan, S.-Y.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.-H.; WULANDARI; Lv, L.-S.; Zhang, Q.-T. Ultrasonic pretreatment improves the stability of zein and Pickering emulsion. Food Ferment Ind. 2021, 47, 97–106. [Google Scholar]
- de Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter. 2012, 8, 6807–6815. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-J.; Ma, C.-C.; Cui, F.-Z.; McClements, D.J.; Liu, X.-B.; Liu, F.-G. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Design and characterization of double-cross-linked emulsion gels using mixed biopolymers: Zein and sodium alginate. Food Hydrocoll. 2021, 113, 106473. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Waterhouse, G.I.N.; Li, D.; Huang, Q. Exploiting the robust network structure of zein/low-acyl gellan gum nanocomplexes to create Pickering emulsion gels with favorable properties. Food Chem. 2021, 349, 129112. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.-J.; Adhikari, B. The effect of addition of flaxseed gum on the emulsion properties of soybean protein isolate (SPI). J. Food Eng. 2011, 104, 56–62. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.-H.; Wang, Y.; Reaney, M.J.T. Whey protein isolate and flaxseed (Linum usitatissimum L.) gum electrostatic coacervates: Turbidity and rheology. Food Hydrocoll. 2017, 64, 18–27. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Tse, T.J.; Wang, Y.; Reaney, M.J.T. Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol. 2018, 75, 146–157. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.-J.; Li, D.; Özkan, N.; Chen, X.D.; Mao, Z.-H. Effect of flaxseed gum addition on rheological properties of native maize starch. J. Food Eng. 2008, 89, 87–92. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Truong, T.; Adhikari, B. Microencapsulation of flaxseed oil using polyphenol-adducted flaxseed protein isolate-flaxseed gum complex coacervates. Food Hydrocoll. 2020, 107, 105944. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Z.-L.; Zhu, J.-Y.; Yin, S.-W.; Tang, C.-H.; Wu, L.-Y.; Yang, X.-Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.-X.; Wei, Y.; Mao, L.; Gao, Y.-X. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.-W.; Meng, X.-H.; Zhang, X.-Y.; Kennedy, J.F.; Liu, B.-J. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef]
- Lv, P.-F.; Wang, D.; Chen, Y.-L.; Zhu, S.-X.; Zhang, J.-B.; Mao, L.-K.; Gao, Y.-X.; Yuan, F. Pickering emulsion gels stabilized by novel complex particles of high-pressure-induced WPI gel and chitosan: Fabrication, characterization and encapsulation. Food Hydrocoll. 2020, 108, 105992. [Google Scholar] [CrossRef]
- Gibis, M.; Rahn, N.; Weiss, J. Physical and oxidative stability of uncoated and chitosan-coated liposomes containing grape seed extract. Pharmaceutics 2013, 5, 421–433. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emamdjomeh, Z.; Razavi, S.H.; Moosavimovahedi, A.A.; Saboury, A.A.; Atri, M.S.; Paul, V.D.M. β-Lactoglobulin–sodium alginate interaction as affected by polysaccharide depolymerization using high intensity ultrasound. Food Hydrocoll. 2013, 32, 235–244. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.-J.; Peng, Q.; Shan, Y.-Y.; Xue, F. Physicochemical properties of peanut protein isolate–glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; McClements, D.J.; Zhou, W.; Peng, S.-F.; Zhou, L.; Zou, L.-Q.; Liu, W. Influence of ionic strength and thermal pretreatment on the freeze-thaw stability of Pickering emulsion gels. Food Chem. 2020, 303, 125401. [Google Scholar] [CrossRef]
- Du, Q.; Tang, J.; Xu, M.; Lyu, F.; Zhang, J.; Qiu, Y.; Liu, J.; Ding, Y. Whey protein and maltodextrin-stabilized oil-in-water emulsions: Effects of dextrose equivalent. Food Chem. 2021, 339, 128094. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.-N.; Bi, S.; Qi, B.-K.; Wang, Z.-J.; Zhang, M.; Li, Y.; Jiang, L.-Z. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Cui, R.-B.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications—ScienceDirect. Trends Food Sci. Technol. 2020, 107, 491–508. [Google Scholar] [CrossRef]
- Ma, X.-B.; Yan, T.-Y.; Hou, F.-R.; Chen, W.-J.; Miao, S.; Liu, D.-H. Formation of soy protein isolate (SPI)-citrus pectin (CP) electrostatic complexes under a high-intensity ultrasonic field: Linking the enhanced emulsifying properties to physicochemical and structural properties. Ultrason. Sonochem. 2019, 59, 104748. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Hu, H.; Cheung, I.W.Y.; Pan, S.Y.; Li-Chan, E.C.Y. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar] [CrossRef]
- Wu, W.-H.; Kong, X.-Z.; Zhang, C.-M.; Hua, Y.-F.; Chen, Y.-M. Improving the stability of wheat gliadin nanoparticles—Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.-L.; Wang, K.; Yagoub, A.E.-G.A.; Owusu, J.; Qu, W.-J.; He, R.-H.; Zhou, C.-S.; Ye, X.-F. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yin, S.-W.; Wu, L.-Y.; Qi, J.-R.; Guo, J.; Yang, X.-Q. Fabrication and characterization of Pickering emulsions and oil gels stabilized by highly charged zein/chitosan complex particles (ZCCPs). Food Chem. 2016, 213, 462–469. [Google Scholar] [CrossRef]
- Hu, C.; Xiong, Z.-Y.; Xiong, H.-G.; Chen, L.; Zhang, Z.-L. Effects of dynamic high-pressure microfluidization treatment on the functional and structural properties of potato protein isolate and its complex with chitosan. Food Res. Int. 2021, 140, 109868. [Google Scholar] [CrossRef]
- Delahaije, R.J.; Gruppen, H.; Giuseppin, M.L.; Wierenga, P.A. Quantitative description of the parameters affecting the adsorption behaviour of globular proteins. Colloids Surf. B 2014, 123, 199–206. [Google Scholar] [CrossRef]
- O’Sullivan, J.J.; Park, M.; Beevers, J.; Greenwood, R.W.; Norton, I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: A review. Food Hydrocoll. 2016, 71, 299–310. [Google Scholar] [CrossRef]
- Jambrak, A.R.E.; Herceg, Z.; Ubari, D.; Babi, J.; Brni, M.; Brni, S.R.; Bosiljkov, T.; ?Vek, D.; Tripalo, B.; Gelo, J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Raoufi, N.; Kadkhodaee, R.; Fang, Y.; Phillips, G.O. Ultrasonic degradation of Persian gum and gum tragacanth: Effect on chain conformation and molecular properties. Ultrason. Sonochem. 2019, 52, 311–317. [Google Scholar] [CrossRef]
- Wang, C.-N.; Wang, H.; Sun, X.-M.; Sun, Y.-X.; Guo, M.-R. Heat-Induced Interactions between Whey Protein and Inulin and Changes in Physicochemical and Antioxidative Properties of the Complexes. Int. J. Mol. Sci. 2019, 20, 4089. [Google Scholar] [CrossRef] [Green Version]
- Mounsey, J.S.; O’Kennedy, B.T.; Fenelon, M.A.; Brodkorb, A. The effect of heating on β-lactoglobulin–chitosan mixtures as influenced by pH and ionic strength. Food Hydrocoll. 2008, 22, 65–73. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.-Z.; Xie, Q.-T.; Cheng, J.-S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Koosis, A.O.; Wang, Q.L.; True, A.D.; Xiong, Y.L.L. Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem. 2021, 350, 129271. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Ahmed, E.; Hu, T.; Xu, X.-Y.; Pan, S.-Y.; Hu, H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason. Sonochem. 2019, 58, 104627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Zhu, X.-F.; Liu, T.-X.; Lin, W.-F.; Tang, C.-H.; Liu, R. Improving freeze-thaw stability of soy nanoparticle-stabilized emulsions through increasing particle size and surface hydrophobicity. Food Hydrocoll. 2019, 87, 404–412. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Cui, Q.; Zhou, M.; Wang, X.-B.; Zhao, X.-H. Improving freeze–thaw stability of soy protein isolate-glucosamine emulsion by transglutaminase glycosylation. Food Bioprod. Process. 2021, 128, 77–83. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; Augustin, M.A. Deamidated wheat protein–dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocoll. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhang, A.-Q.; Wang, X.-B.; Xu, N.; Jiang, L.-Z. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]











| Sample No. | Complexes | Materials | Process | ||
|---|---|---|---|---|---|
| Protein | Polysaccharides | Homogenizing (rpm/3 min) | Ultrasound (W·cm−2) | ||
| 1 | ZNP | ZNP | - | - | - |
| 2 | ZNPU | ZNPU | - | - | 52.95 |
| 3 | FSG | - | FSG | - | - |
| 4 | FSGU | - | FSGU | - | 52.95 |
| 5 | (ZNP-FSG)H | ZNP | FSG | 15,000 | - |
| 6 | (ZNPU-FSG)H | ZNPU | FSG | 15,000 | 52.95 |
| 7 | (ZNP-FSGU)H | ZNP | FSGU | 15,000 | 52.95 |
| 8 | (ZNP-FSG)HU | ZNP | FSG | 15,000 | 52.95 |
| 9 | (ZNP-FSG)U | ZNP | FSG | - | 52.95 |
| Creaming Index (%) | Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 2 h | 1 d | 2 d | 3 d | 4 d | 7 d | 10 d | 14 d | |
| ZNP-E | 25 | 25 | 25 | 27.5 | 30 | 30 | 30 | 30 | 30 |
| (ZNP-FSG)H-E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (ZNPU-FSG)H-E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (ZNP-FSGU)H-E | 7.5 | 7.5 | 8 | 10 | 10 | 10 | 25 | 25 | 25 |
| (ZNP-FSG)HU-E | 17.5 | 22.5 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| (ZNP-FSG)U-E | 17.5 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, G.; Li, W.; Lv, L.; Zhang, Q. The Role of Ultrasound in the Preparation of Zein Nanoparticles/Flaxseed Gum Complexes for the Stabilization of Pickering Emulsion. Foods 2021, 10, 1990. https://doi.org/10.3390/foods10091990
Li Y, Xu G, Li W, Lv L, Zhang Q. The Role of Ultrasound in the Preparation of Zein Nanoparticles/Flaxseed Gum Complexes for the Stabilization of Pickering Emulsion. Foods. 2021; 10(9):1990. https://doi.org/10.3390/foods10091990
Chicago/Turabian StyleLi, Yinghao, Ge Xu, Weiwei Li, Lishuang Lv, and Qiuting Zhang. 2021. "The Role of Ultrasound in the Preparation of Zein Nanoparticles/Flaxseed Gum Complexes for the Stabilization of Pickering Emulsion" Foods 10, no. 9: 1990. https://doi.org/10.3390/foods10091990
APA StyleLi, Y., Xu, G., Li, W., Lv, L., & Zhang, Q. (2021). The Role of Ultrasound in the Preparation of Zein Nanoparticles/Flaxseed Gum Complexes for the Stabilization of Pickering Emulsion. Foods, 10(9), 1990. https://doi.org/10.3390/foods10091990