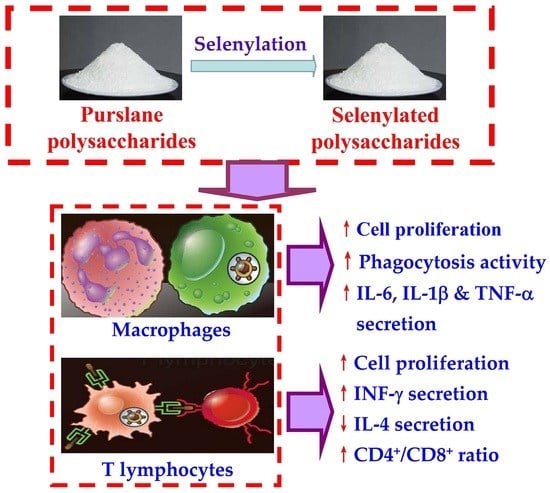
In Vitro Immuno-Modulatory Potentials of Purslane (Portulaca oleracea L.) Polysaccharides with a Chemical Selenylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal and Cells
2.3. Polysaccharide Extraction
2.4. PSPO Selenylation and Se Detection
2.5. Assays of Cell Viability and Phagocytic Activity of the Macrophages
2.6. Assays of Proliferation of the Polysaccharide Samples on Murine Splenocytes
2.7. Assays of Cytokine Secretion in the Macrophages and Murine Splenocytes
2.8. Assays of T Lymphocyte Subpopulations
2.9. Statistical Analysis
3. Results
3.1. Macrophage Proliferation and Phagocytosis as Affected by the PSPO and SePSPO
3.2. Cytokine Secretion of the Macrophages as Affected by the PSPO and SePSPO
3.3. Splenocyte Proliferation as Affected by the PSPO and SePSPO
3.4. Cytokine Secretion of the Murine Splenocytes as Affected by the PSPO and SePSPO
3.5. T lymphocyte Subpopulations as Affected by the PSPO and SePSPO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 2020, 320, e126621. [Google Scholar] [CrossRef] [PubMed]
- Alfwuaires, M.A.; Algefare, A.I.; Afkar, E.; Salam, S.A.; Abd El-Moaty, H.I.; Badr, G.M. Immunomodulatory assessment of Portulaca oleracea L. extract in a mouse model of colitis. Biomed. Pharmacother. 2021, 143, e112148. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.E.A.; Dkhil, M.A.; Al-Quraishy, S. The potential role of Portulaca oleracea as a neuroprotective agent in rotenone-induced neurotoxicity and apoptosis in the brain of rats. Pestic. Biochem. Physiol. 2013, 105, 203–212. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Bai, Y.; Zang, X.L.; Ma, J.S.; Xu, G.Y. Anti-diabetic effect of portulaca oleracea L. polysaccharide and its mechanism in diabetic rats. Int. J. Mol. Sci. 2016, 17, 1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Bai, Y.P.; Zhang, Z.; Cai, W.L.; Flores, A.D. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.L.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.J.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]
- Yuan, R.S.; Tao, X.; Liang, S.; Pan, Y.; Li, H.; Sun, J.H.; Ju, W.B.; Li, X.Y.; Chen, J.G.; Wang, C.M. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T.; Huang, X.J.; Wang, S.N.; Li, C.; Zhang, Z.H.; Xie, M.Y.; Nie, S.P. Combinatorial usage of fungal polysaccharides from Cordyceps sinensis and Ganoderma atrum ameliorate drug-induced liver injury in mice. Food Chem. Toxicol. 2018, 119, 66–72. [Google Scholar] [CrossRef]
- He, N.W.; Zhai, X.C.; Zhang, X.B.; Zhang, X.W.; Wang, X.J. Extraction, purification and characterization of water-soluble polysaccharides from green walnut husk with anti-oxidant and anti-proliferative capacities. Process Biochem. 2020, 88, 170–179. [Google Scholar] [CrossRef]
- Li, G.Q.; Chen, P.F.; Zhao, Y.T.; Zeng, Q.H.; Ou, S.Y.; Zhang, Y.H.; Wang, P.C.; Chen, N.H.; Ou, J.Y. Isolation, structural characterization and anti-oxidant activity of a novel polysaccharide from garlic bolt. Carbohydr. Polym. 2021, 267, e118194. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.H.; Zheng, Y.F.; Xu, Z.C.; Li, Y.Q.; Zhou, S.; Zhang, C.S. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, e110182. [Google Scholar] [CrossRef]
- Lopez-Legarda, X.; Arboleda-Echavarria, C.; Parra-Saldivar, R.; Rostro-Alanis, M.; Alzate, J.F.; Villa-Pulgarin, J.A.; Segura-Sanchez, F. Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int. J. Biol. Macromol. 2020, 164, 3133–3144. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Xiang, Y.F.; Sheng, R.L.; Tomas, H.; Rodrigues, J.; Gu, Z.W.; Zhang, H.; Gong, Q.Y.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, W.Y.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.M.; Wang, K.P. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Q.; Hu, Y.K.; Shi, S.J.; Jiang, L. Evaluation of antioxidant and immuno-enhancing activities of purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.P.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Guo, H.J.; Chen, C.Y.; Yan, X.; Li, Y.Y.; Wen, X.B.; You, C.H.; Monroig, O.; Tocher, D.R.; Wang, S.Q. Effects of different dietary oil sources on growth performance, antioxidant capacity and lipid deposition of juvenile golden pompano Trachinotus ovatus. Aquaculture 2021, 530, e735923. [Google Scholar] [CrossRef]
- Cai, J.Y.; Li, X.; Du, H.M.; Jiang, C.F.; Xu, S.L.; Cao, Y. Immunomodulatory significance of natural peptides in mammalians: Promising agents for medical application. Immunobiology 2020, 225, e151936. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Diaz, A.; Gonzalez-Cordova, A.F.; Hernandez-Mendoza, A.; Reyes-Diaz, R.; Vallejo-Cordoba, B. Immunomodulation by hydrolysates and peptides derived from milk proteins. Int. J. Dairy Technol. 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Yang, J.X.; Maria, T.C.; Zhou, B.; Xiao, F.L.; Li, Y. Quercetin improves immune function in Arbor Acre broilers through activation of NF-κB signaling pathway. Poult. Sci. 2020, 99, 906–913. [Google Scholar] [CrossRef]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular metabolism turns immune regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.Y.; Li, F.L.; Zhang, L.L.; Li, J.; Zhang, Z.; Wang, G.Y. Hypoglycemic effects of crude polysaccharide from purslane. Int. J. Mol. Sci. 2009, 10, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, Z.; Chen, X. Evaluation of free radicals scavenging and immunity-modulatory activities of purslane polysaccharides. Int. J. Biol. Macromol. 2009, 45, 448–452. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Jagtap, R.; Maher, W. Determination of selenium species in biota with an emphasis on animal tissues by HPLC-ICP-MS. Microchem. J. 2016, 124, 422–529. [Google Scholar] [CrossRef]
- Li, G.R.; Xiang, Y.; Zhao, J.; Chang, J.M. Saccharum Alhagi polysaccharide-1 and -2 promote the immunocompetence of RAW 264.7 macrophages In Vitro. Exp. Ther. Med. 2018, 15, 3556–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, G.F.; Xie, Z.W.; Huang, S.X.; Tai, Y.L.; Cai, Q.S.; Jiang, W.; Sun, J.M.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wang, C.L.; Wang, Y.R.; Li, Z.J.; Zhang, Y.N. The polysaccharide isolated from Pleurotus nebrodensis (PN-S) shows immune-stimulating activity in RAW 264.7 macrophages. Chin. J. Nat. Med. 2015, 13, 355–360. [Google Scholar] [CrossRef]
- Wang, C.L.; Cui, H.Y.; Wang, Y.R.; Wang, Z.F.; Li, Z.J.; Chen, M.H.; Li, F.J. Bidirectional immunomodulatory activities of polysaccharides purified from Pleurotus nebrodensis. Inflammation 2014, 37, 83–93. [Google Scholar] [CrossRef]
- Silva, M.S.D.; Santos, J.D.; Alves, A.J.; da Silva, R.M.F.; Santos, B.S.; de Lorena, V.M.B.; de Oliveira, G.G.; de Melo, C.M.L.; Goes, A.J.D. Evaluation of the immunomodulatory effect against splenocytes of Balb/c mice of biflorin obtained from Capraria biflora by a new isolation method. Rev. Bras. Farmacogn.-Braz. J. Pharmacogn. 2019, 29, 464–469. [Google Scholar] [CrossRef]
- Zhao, H.J.; Zhao, X.H. Effect of the Zn supplementation on immuno-modulatory activities of bovine lactoferrin in the murine splenocytes and RAW 264.7 macrophages. Biol. Trace Elem. Res. 2019, 192, 287–296. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wan, D.L.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Structural characteristics and immunostimulatory activities of a new polysaccharide from Dendrobium fimbriatum Hook. Food Funct. 2021, 12, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Bangkok, Thailand, 2004; pp. 194–216. [Google Scholar]
- Li, Y.; Deng, Y.; Li, Z.; Liu, Z.Q.; Piao, M.Z.; Cui, X.Q. Composition, physicochemical properties, and anti-fatigue activity of water-soluble okra (Abelmoschus esculentus) stem pectins. Int. J. Biol. Macromol. 2020, 165, 2630–2639. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Zhang, W.C.; Wu, Z.Y.; Liu, R.; Yang, C.Y.; Hui, A.L.; Huang, X.S.; Xian, Z.J. Preparation, characterization, anti-oxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.F.; Hu, X.B.; Wang, J.H. Structural characterization and anti-inflammatory activity of a polysaccharide from the lignified okra. Carbohydr. Polym. 2021, 265, e118081. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, e129437. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, D.; Sadat, A.; Dam, P.; Buccini, D.F.; Mandal, A.K. Current concepts and prospects of mulberry fruits for nutraceutical and medicinal benefits. Curr. Opin. Food Sci. 2021, 40, 121–135. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Hu, D.W.; Mo, J.L.; Ni, J.D. Black mulberry (Morus nigra L.) polysaccharide ameliorates palmitate-induced lipotoxicity in hepatocytes by activating Nrf2 signaling pathway. Int. J. Biol. Macromol. 2021, 172, 394–407. [Google Scholar] [CrossRef]
- Cheng, K.C.; Wang, C.J.; Chang, Y.C.; Hung, T.W.; Lai, C.J.; Kuo, C.W.; Huang, H.P. Mulberry fruits extracts induce apoptosis and autophagy of liver cancer cell and prevent hepatocarcinogenesis in vivo. J. Food Drug Anal. 2020, 28, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.G.; Jiang, W.; Nitin, M.; Bao, X.Q.; Chen, S.L.; Tao, Z.M. Characterizing diversity based on nutritional and bioactive compositions of yam germplasm (Dioscorea spp.) commonly cultivated in China. J. Food Drug Anal. 2016, 24, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.Y.; Hu, M.; Tao, J.; Yang, H.; Yan, P.J.; An, G.P.; Wang, H.L. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Hao, L.X.; Zhao, X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016, 27, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Xie, J.H.; Yu, Y.; Shen, M.Y. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Wu, J.Q.; Peng, W.; Qin, R.X.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Yu, J.C.; Fu, M.F.; Wang, X.F.; Chang, X.D. Regulatory effects of hawthorn polyphenols on hyperglycemic, inflammatory, insulin resistance responses, and alleviation of aortic injury in type 2 diabetic rats. Food Res. Int. 2021, 142, e110239. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Szczypka, M.; Suszko-Pawlowska, A.; Sokol-Letowska, A.; Kucharska, A.; Obminska-Mrukowicz, B. Hawthorn (Crataegus monogyna) phenolic extract modulates lymphocyte subsets and humoral immune response in mice. Planta Med. 2020, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Z.; Li, S.W.; Ma, Y.; Hu, D.; Xiao, F. Curcumin hinders PBDE-47-induced neutrophil extracellular traps release via Nrf2-associated ROS inhibition. Ecotoxicol. Environ. Saf. 2021, 225, e112779. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Su, X.; Zhao, M.; Fu, X.; Ma, X.; Xu, W.; Hu, S. Immunomodulatory activity of purified polysaccharides from Rubus chingii Hu fruits in lymphocytes and its molecular mechanisms. J. Funct. Foods 2021, 87, e104785. [Google Scholar] [CrossRef]
- Li, R.; Qin, X.J.; Liu, S.; Zhang, X.Y.; Zeng, X.R.; Guo, H.Y.; Wang, T.; Zhang, Y.D.; Zhang, J.P.; Zhang, J.; et al. HNMP HSO4 catalyzed synthesis of selenized polysaccharide and its immunomodulatory effect on RAW 264.7 cells via MAPKs pathway. Int. J. Biol. Macromol. 2020, 160, 1066–1077. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Huang, Y.; Song, Y.; Lin, D.; Li, J.; Ma, Y.; Wu, X.; Qiu, F.; Xiao, Q. Selenizing Hericium erinaceus polysaccharides induces dendritic cells maturation through MAPK and NF-kappa B signaling pathways. Int. J. Biol. Macromol. 2017, 97, 287–298. [Google Scholar] [CrossRef]
- Li, S.J.; Xiong, Q.P.; Lai, X.P.; Li, X.; Wan, M.; Zhang, J.N.; Yan, Y.J.; Cao, M.; Lu, L.; Guan, J.M.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Bao, A.J.; Meng, X.H.; Guo, H.Y.; Zhang, Y.D.; Zhao, Y.L.; Kong, W.B.; Liang, J.Y.; Yao, J.; Zhang, J. An efficient approach to prepare sulfated polysaccharide and evaluation of anti-tumor activities in vitro. Carbohydr. Polym. 2018, 184, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Chen, J.L.; Lei, L.; Li, F.H.; Tang, Y.; Yuan, Y.; Zhang, Y.Q.; Wu, S.R.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW 264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Shen, M.Y.; Tang, W.; Wang, H.; Nie, S.P.; Xie, M.Y. Carboxymethylation of polysaccharide from Cyclocarya paliurus and their characterization and antioxidant properties evaluation. Carbohydr. Polym. 2016, 136, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Fan, J.; Yang, S.P.; Zhao, X.L.; Yi, X. Antiviral activity of phosphorylated Radix Cyathulae officinalis polysaccharide against canine parvovirus in vitro. Int. J. Biol. Macromol. 2017, 99, 511–518. [Google Scholar] [CrossRef]
- Cheng, L.Z.; Wang, Y.F.; He, X.X.; Wei, X.L. Preparation, structural characterization and bioactivities of Se-containing polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 82–92. [Google Scholar] [CrossRef]
- Gao, P.Y.; Bian, J.; Xu, S.S.; Liu, C.F.; Sun, Y.Q.; Zhang, G.L.; Li, D.Q.; Liu, X.G. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef] [PubMed]




| Cell Group | Dose (μg/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|
| Control | None | 10.69 ± 1.24 f | 1.55 ± 0.40 f | 61.39 ± 1.70 g |
| PSPO | 5 | 13.85 ± 1.26 e | 2.87 ± 0.61 f | 68.78 ± 1.20 f |
| 20 | 33.81 ± 0.69 c | 5.48 ± 1.05 e | 112.98 ± 1.78 c | |
| SePSPO-1 | 5 | 22.30 ± 1.40 d | 6.29 ± 0.60 c | 76.76 ± 1.40 e |
| 20 | 42.52 ± 0.24 b | 7.73 ± 0.45 bc | 128.96 ± 1.55 b | |
| SePSPO-2 | 5 | 32.81 ± 1.86 c | 8.53 ± 0.99 b | 86.90 ± 0.97 d |
| 20 | 47.58 ± 1.61 a | 11.71 ± 0.77 a | 144.16 ± 3.49 a |
| Cell Group | Dose (μg/mL) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ Ratio |
|---|---|---|---|---|
| Control | None | 29.7 ± 1.2 | 14.4 ± 0.6 | 2.06 ± 0.01 |
| PSPO | 5 | 30.8 ± 2.0 | 14.8 ± 1.1 | 2.08 ± 0.02 |
| 20 | 31.9 ± 2.8 | 14.5 ± 1.3 | 2.20 ± 0.59 | |
| SePSPO-1 | 5 | 31.9 ± 1.8 | 14.6 ± 0.8 | 2.18 ± 0.01 |
| 20 | 32.3 ± 1.9 | 14.2 ± 0.7 | 2.28 ± 0.06 | |
| SePSPO-2 | 5 | 30.8 ± 0.9 | 13.6 ± 0.7 | 2.27 ± 0.08 |
| 20 | 34.1 ± 3.3 | 14.1 ± 1.1 | 2.41 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-R.; Guan, Q.-Y.; Li, L.-Y.; Tang, Z.-M.; Zhang, Q.; Zhao, X.-H. In Vitro Immuno-Modulatory Potentials of Purslane (Portulaca oleracea L.) Polysaccharides with a Chemical Selenylation. Foods 2022, 11, 14. https://doi.org/10.3390/foods11010014
Lin Y-R, Guan Q-Y, Li L-Y, Tang Z-M, Zhang Q, Zhao X-H. In Vitro Immuno-Modulatory Potentials of Purslane (Portulaca oleracea L.) Polysaccharides with a Chemical Selenylation. Foods. 2022; 11(1):14. https://doi.org/10.3390/foods11010014
Chicago/Turabian StyleLin, Ya-Ru, Qing-Yun Guan, Ling-Yu Li, Zhi-Mei Tang, Qiang Zhang, and Xin-Huai Zhao. 2022. "In Vitro Immuno-Modulatory Potentials of Purslane (Portulaca oleracea L.) Polysaccharides with a Chemical Selenylation" Foods 11, no. 1: 14. https://doi.org/10.3390/foods11010014
APA StyleLin, Y. -R., Guan, Q. -Y., Li, L. -Y., Tang, Z. -M., Zhang, Q., & Zhao, X. -H. (2022). In Vitro Immuno-Modulatory Potentials of Purslane (Portulaca oleracea L.) Polysaccharides with a Chemical Selenylation. Foods, 11(1), 14. https://doi.org/10.3390/foods11010014