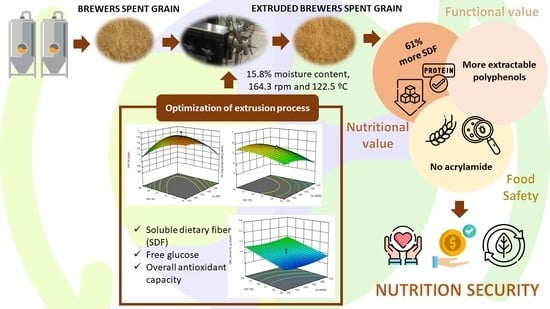
Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Raw Material
2.3. Optimization of Extrusion Process
2.4. Nutritional Characterization
2.4.1. Dietary Fiber Content
2.4.2. Starch Content
2.4.3. Free Glucose Content
2.4.4. Protein Content and Amino Acid Analysis
- The protein efficiency ratio (PER) was based on the following three equations [24]:
- The essential amino acid index (EAAI) was calculated as the geometrical mean of the ratio of all the EAA in the evaluated food matrix to their content in a highly nutritive reference protein such as whole egg [27].
2.4.5. Fat Content
2.4.6. Ash Content
2.5. Functional Characterization
2.5.1. Antioxidant´s Extraction
2.5.2. Extractable Phenolic Content (EPC)
2.5.3. Antioxidant Capacity of the Extractable Compounds
2.5.4. Analysis of Phenolic Compounds by HPLC-QTOF Assay
2.5.5. Melanoidin Content
2.6. Food Safety Analysis
2.6.1. Microbiological Quality
2.6.2. Gluten Content
2.6.3. Acrylamide Content
2.7. Statistical Data Analysis
3. Results and Discussion
3.1. Optimization of Extrusion Process
3.1.1. Statistical Analysis and Model Fitting
3.1.2. Soluble Dietary Fiber (SDF)
3.1.3. Free Glucose (FG)
3.1.4. Overall Antioxidant Capacity (OAC)
3.1.5. Multiple Response Desirability Optimization of BSG Extrusion Conditions
3.2. Nutritional Characterization
3.3. Functional Characterization
3.4. Food Safety Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/OMS Dietas Saludables Sostenibles-Principios Rectores; 2020; ISBN 9789251328453. Available online: https://www.fao.org/3/ca6640es/CA6640ES.pdf (accessed on 17 March 2022).
- Mozaffarian, D.; Fleischhacker, S.; Andrés, J. Prioritizing Nutrition Security in the US. J. Am. Med. Assoc. 2021, 02111, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’ s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Sanchez Lopez, J.; Patinha Caldeira, C.; De Laurentiis, V.; Sala, S.; Avraamides, M. Brief on Food Waste in the European Union. Available online: https://joint-research-centre.ec.europa.eu/publications/brief-food-waste-european-union_en (accessed on 17 March 2022).
- Rumaihi, A.A.; Mckay, G.; Mackey, H.R.; Ansari, T. Al Environmental Impact Assessment of Food Waste Management Using Two Composting Techniques. Sustainability 2020, 12, 1595. [Google Scholar] [CrossRef] [Green Version]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current Extraction Techniques towards Bioactive Compounds from Brewer’ s Spent Grain—A Review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2730–2741. [Google Scholar] [CrossRef]
- Jaglo, K.; Kenny, S.; Stephenson, J. From Farm to Kitchen: The Environmental Impacts of US. Food Waste. Available online: https://www.epa.gov/system/files/documents/2021-11/from-farm-to-kitchen-the-environmental-impacts-of-u.s.-food-waste_508-tagged.pdf (accessed on 17 March 2022).
- FAO Sustainable Diets and Biodiversity: Directions and Solutions for Policy, Research and Action; Rome, 2012. Available online: https://www.fao.org/3/i3004e/i3004e.pdf (accessed on 17 March 2022).
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. Inst. Brew. Distill. 2016, 553–568. [Google Scholar] [CrossRef]
- Steiner, J.; Procopio, S. Brewer’ s Spent Grain: Source of Value Added Polysaccharides for the Food Industry in Reference to the Health Claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedzwiecki, L.; Jagiello, K.; Uchanska, O.; Trusek, A. Brewer’ s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Mccarthy, A.L.; Callaghan, Y.C.O.; Piggott, C.O.; Fitzgerald, R.J.; Brien, N.M.O. Brewers’ Spent Grain; Bioactivity of Phenolic Component, Its Role in Animal Nutrition and Potential for Incorporation in Functional Foods: A Review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Adekola, K.A. Engineering Review Food Extrusion Technology and Its Applications. J. Food Sci. Eng. 2016, 6, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Obradović, V.; Babic, J.; Šubarić, D.; Ačkar, D.; Jozinović, A. Improvement of Nutritional and Functional Properties of Extruded Food Products Improvement of Nutritional and Functional Properties of Extruded Food Products. J. Food Nutr. Res. 2014, 53, 189–206. [Google Scholar]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Zhang, R.; Khan, S.A.; Chi, J.; Wei, Z.; Zhang, Y.; Deng, Y.; Liu, L.; Zhang, M. Different Effects of Extrusion on the Phenolic Profiles and Antioxidant Activity in Milled Fractions of Brown Rice. LWT Food Sci. Technol. 2017, 88, 64–70. [Google Scholar] [CrossRef]
- Björck, I.; Asp, N.G. The Effects of Extrusion Cooking on Nutritional Value—A Literature Review. J. Food Eng. 1983, 2, 281–308. [Google Scholar] [CrossRef]
- Natabirwa, H.; Nakimbugwe, D.; Lungaho, M.; Muyonga, J.H. Optimization of Roba1 Extrusion Conditions and Bean Extrudate Properties Using Response Surface Methodology and Multi-Response Desirability Function. LWT 2018, 96, 411–418. [Google Scholar] [CrossRef]
- Ramos-Enríquez, J.R.; Ramírez-Wong, B.; Robles-Sánchez, R.M.; Robles-Zepeda, R.E.; González-Aguilar, G.A.; Gutiérrez-Dorado, R. Effect of Extrusion Conditions and the Optimization of Phenolic Compound Content and Antioxidant Activity of Wheat Bran Using Response Surface Methodology. Plant Foods Hum. Nutr. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Protonotariou, S.; Mandala, I.; Rosell, C.M. Jet Milling Effect on Functionality, Quality and In Vitro Digestibility of Whole Wheat Flour and Bread. Food Bioprocess Technol. 2015, 8, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct Measurement of the Total Antioxidant Capacity of Foods: The “QUENCHER” Approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012.
- Spackman, D.; Stein, W.; Moore, S. Automatic Recording Apparatus for Use in the Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of PH and Holding Time on the Characteristics of Protein Isolates from Chenopodium Seeds and Study of Their Amino Acid pro Fi Le and Scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef]
- Volk, C.; Brandsch, C.; Schlegelmilch, U.; Wensch-dorendorf, M.; Hirche, F.; Simm, A.; Gargum, O.; Wiacek, C.; Braun, P.G.; Kopp, J.F.; et al. Postprandial Metabolic Response to Rapeseed Protein in Healthy Subjects. Nutrients 2020, 12, 2270. [Google Scholar] [CrossRef]
- Greco, E.; Winquist, A.; Lee, T.J.; Collins, S.; Lebovic, Z.; Kessinger, T.Z.; Mihan, A.J. The Role of Source of Protein in Regulation of Food Intake, Satiety, Body Weight and Body Composition. J. Nutr. Health Food Eng. 2017, 6, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins; Academic Press Inc., Ed.; Academic Press, Inc.: New York, NY, USA, 1959. [Google Scholar]
- Ijarotimi Oluwole, S. Influence of Germination and Fermentation on Chemical Composition, Protein Quality and Physical Properties of Wheat Flour (Triticum Aestivum). J. Cereals Oilseeds 2012, 3, 35–47. [Google Scholar] [CrossRef]
- Bodwell, C. FAO/WHO/UNU Various Comments Related to Amino Acid Scoring Patterns. Available online: https://www.fao.org/3/AA052E/AA052E00.htm (accessed on 14 February 2022).
- Fernández, A.; Iriondo-dehond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano, A.; del Castillo, M.D. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.S.; Obem, C.; Caetano, V.; Paula, A.; Fruet, B.; Bromenberg, M.; Cristina, A.; Laerte, J. Analysis of Polyphenols in Brewer’ s Spent Grain and Its Comparison with Corn Silage and Cereal Brans Commonly Used for Animal Nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-Dehond, A.; García, N.A.; Fernandez-gomez, B.; Guisantes-batan, E.; Velázquez, F.; Patricia, G.; Ignacio, M.; Andres, S.; Sanchez-fortun, S.; Dolores, M. Validation of Co Ff Ee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R. Determination of Total Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe: First Action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Patrignani, M.; González-Forte, L.S. Characterisation of Melanoidins Derived from Brewers’ Spent Grain: New Insights into Their Structure and Antioxidant Activity. Int. J. Food Sci. Technol. 2020, 56, 384–391. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Ramírez, B.; Velazquez Escobar, F.; del Castillo, M.D. Antioxidant Properties of High Molecular Weight Compounds from Coffee Roasting and Brewing Byproducts. Bioact. Compd. Heal. Dis. 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Mena, M.C.; Lombardía, M.; Hernando, A.; Méndez, E.; Albar, J.P. Comprehensive Analysis of Gluten in Processed Foods Using a New Extraction Method and a Competitive ELISA Based on the R5 Antibody. Talanta 2012, 91, 33–40. [Google Scholar] [CrossRef]
- Ehotay, S.T.J.L. Rapid Sample Preparation Method for LC−MS/MS or GC−MS Analysis of Acrylamide in Various Food Matrices AND. J. Agric. Food Chem. 2006, 54, 7001–7008. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S. Optimization of the Extrusion Process for Preparation of Soluble Dietary Fiber-Enriched Calamondin Pomace and Its Influence on the Properties of Bread. J. Food Sci. Technol. 2019, 56, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chi, Y.J. Effects of Twin-Screw Extrusion on Soluble Dietary Fibre and Physicochemical Properties of Soybean Residue. Food Chem. 2013, 138, 884–889. [Google Scholar] [CrossRef]
- Sandrin, R.; Mejía, S.M.V.; Caon, T.; de Francisco, A. Optimization of Extrusion Process Parameters for Preparing Fiber-Rich Oat Flour. J. Food Process Eng. 2019, 42, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Larrea, M.A.; Chang, Y.K.; Mart, F. Effect of Some Operational Extrusion Parameters on the Constituents of Orange Pulp. Food Chem. 2005, 89, 301–308. [Google Scholar] [CrossRef]
- Martínez, M.M.; Calviño, A.; Rosell, C.M.; Gómez, M. Effect of Different Extrusion Treatments and Particle Size Distribution on the Physicochemical Properties of Rice Flour. Food Bioprocess Technol. 2014, 7, 2657–2665. [Google Scholar] [CrossRef] [Green Version]
- Xu, E.; Pan, X.; Wu, Z.; Long, J.; Li, J.; Xu, X.; Jin, Z.; Jiao, A. Response Surface Methodology for Evaluation and Optimization of Process Parameter and Antioxidant Capacity of Rice Flour Modified by Enzymatic Extrusion. Food Chem. 2016, 212, 146–154. [Google Scholar] [CrossRef]
- Soison, B.; Jangchud, K.; Jangchud, A.; Harnsilawat, T.; Piyachomkwan, K. Physico-Functional and Antioxidant Properties of Purple-Flesh Sweet Potato Flours as Affected by Extrusion and Drum-Drying Treatments. Int. J. Food Sci. Technol. 2014, 49, 2067–2075. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J. Protein Content and Amino Acid Composition of Commercially Available Plant Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Iriondo-Dehond, A.; Salazar, M.d.P.; Guspí Domènech, L.; Zapata Montoya, J.E.; del Castillo, M.D. Coffee Proteins: Functional Food Ingredients with Molecular Effects for Sustainable Health. In Molecular Mechanisms of some Functional Foods: The Frontier between Health and Disease; (submitted; accepted; in press); Campos-Vega, R., Oomah, B.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; (submitted; accepted; in press). [Google Scholar]
- Nagata, C.; Nakamura, K.; Wada, K.; Tsuji, M.; Tamai, Y.; Kawachi, T. Branched-Chain Amino Acid Intake and the Risk of Diabetes in a Japanese Community The Takayama Study. Am. J. Epidemiol. 2013, 178, 1226–1232. [Google Scholar] [CrossRef]
- Mohan, N.; Mellem, J.J. Functional Properties of the Protein Isolates of Hyacinth Bean [ Lablab Purpureus (L.) Sweet ]: An e Ff Ect of the Used Procedures. LWT Food Sci. Technol. 2020, 129, 1–8. [Google Scholar] [CrossRef]
- Ivanova, K.; Denkova, R.; Kostov, G.; Petrova, T.; Bakalov, I.; Ruscova, M.; Penov, N. Extrusion of Brewers’ Spent Grains and Application in the Production of Functional Food. Characteristics of Spent Grains and Optimization of Extrusion. Inst. Brew. Distill. 2017, 123, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Zhang, J.; Duan, Y. A Mini-Review on Brewer’ s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Hejna, A.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Chmielnicki, B.; Korol, J.; Formela, K. Sustainable Upcycling of Brewer’s Spent Grain by Thermo-Mechanical Treatment in Twin-Screw Extruder. J. Clean. Prod. 2020, 285, 124839. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of Legumes in Extrusion Cooking: A Review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Igual, M.; Chi, M.S.; Păucean, A.; Vodnar, D.C.; Ranga, F.; Mihăiescu, T.; Török, A.; Fărcas, A.; Martínez-Monzó, J.; García-Segovia, P. Effect on Nutritional and Functional Characteristics by Encapsulating Rose Canina Powder in Enriched Corn Extrudates. Foods 2021, 10, 2401. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-cerio, E.; Coda, R. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef]



| Assay | Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coded | Actual | Responses | |||||||
| X1: MC | X2: SS | X3: BT | X1: MC (%) | X2: SS (rpm) | X3: BT (°C) | Y1: SDF (% dwb) | Y2: FG (mg glucose/g dwb) | Y3: OAC (mmol TE/ g dwb) | |
| 0 | - | - | - | 3 | 0 | 0 | 1.103 ± 0.028 | 1.250 ± 0.028 | 1.315 ± 0.008 |
| 1 | −1 | −1 | −1 | 15 | 125 | 105 | 1.687 ± 0.064 | 5.612 ± 0.047 | 1.276 ± 0.108 |
| 2 | 1 | −1 | −1 | 25 | 125 | 105 | 1.235 ± 0.006 | 5.590 ± 0.032 | 1.094 ± 0.028 |
| 3 | −1 | 1 | −1 | 15 | 175 | 105 | 1.634 ± 0.069 | 5.525 ± 0.171 | 1.324 ± 0.110 |
| 4 | 1 | 1 | −1 | 25 | 175 | 105 | 1.480 ±0.050 | 5.685 ± 0.132 | 1.109 ± 0.046 |
| 5 | −1 | −1 | 1 | 15 | 125 | 155 | 1.388 ± 0.045 | 5.584 ± 0.063 | 1.121 ± 0.070 |
| 6 | 1 | −1 | 1 | 25 | 125 | 155 | 1.233 ± 0.022 | 5.537 ± 0.199 | 1.036 ± 0.038 |
| 7 | −1 | 1 | 1 | 15 | 175 | 155 | 1.341 ± 0.009 | 4.857 ± 0.315 | 1.226 ±0.044 |
| 8 | 1 | 1 | 1 | 25 | 175 | 155 | 1.037 ± 0.012 | 4.279 ± 0.035 | 1.101 ± 0.076 |
| 9 | 0 | 0 | 0 | 20 | 150 | 130 | 1.992 ± 0.095 | 5.826 ± 0.306 | 1.076 ± 0.030 |
| 10 | −1.68 | 0 | 0 | 11.59 | 150 | 130 | 1.338 ± 0.030 | 6.266 ± 0.152 | 1.180 ± 0.026 |
| 11 | 1.68 | 0 | 0 | 28.41 | 150 | 130 | 1.333 ± 0.055 | 3.927 ± 0.150 | 1.027 ± 0.043 |
| 12 | 0 | −1.68 | 0 | 20 | 107.95 | 130 | 1.229 ± 0.063 | 4.710 ± 0.181 | 1.133 ± 0.012 |
| 13 | 0 | 1.68 | 0 | 20 | 192.05 | 130 | 1.255 ± 0.005 | 6.027 ± 0.204 | 1.171 ± 0.030 |
| 14 | 0 | 0 | −1.68 | 20 | 150 | 87.96 | 0.820 ± 0.010 | 5.451 ± 0.035 | 1.252 ± 0.018 |
| 15 | 0 | 0 | 1.68 | 20 | 150 | 172.05 | 0.691 ± 0.019 | 5.649 ± 0.398 | 1.070 ± 0.054 |
| 16 | 0 | 0 | 0 | 20 | 150 | 130 | 1.775 ± 0.043 | 5.471 ± 0.265 | 1.107 ± 0.027 |
| 17 | 0 | 0 | 0 | 20 | 150 | 130 | 1.753 ± 0.084 | 5.803 ± 0.306 | 0.991 ± 0.056 |
| 18 | 0 | 0 | 0 | 20 | 150 | 130 | 1.802 ± 0.070 | 5.595 ± 0.013 | 1.077 ± 0.072 |
| 19 | 0 | 0 | 0 | 20 | 150 | 130 | 1.738 ± 0.059 | 5.564 ± 0.266 | 1.137 ± 0.014 |
| Coefficients in Terms of Coded Factors | |||
|---|---|---|---|
| Factors | Y1: SDF | Y2: FG | Y3: OAC |
| Intercept | 1.81 | 5.44 | 1.10 |
| −0.0383 | −0.4793 *** | −0.0560 *** | |
| +0.0397 | −0.3402 *** | 0.0145 | |
| −0.0515 | 0.1688 *** | −0.0531 *** | |
| −0.2924 ** | −0.1471 *** | ns | |
| −0.2925 ** | 0.3468 *** | ns | |
| −0.3652 *** | −0.1139 ** | 0.0357 ** | |
| −0.1688 *** | −0.2679 *** | ns | |
| −0.2019 *** | −0.3846 *** | 0.0196 * | |
| −0.3740 *** | 0.0798 ** | 0.0226 * | |
| Fit Statistics | |||
| F-value (model) | 20.44 *** | 68.12 *** | 33.93 ** |
| Lack of fit (p-value) | 0.5646 | 0.7886 | 0.8273 |
| R2 | 0.9633 | 0.9082 | 0.9532 |
| Adjusted R2 | 0.9162 | 0.8948 | 0.9251 |
| Predicted R2 | 0.7443 | 0.8743 | 0.8806 |
| CV | 7.07 | 4.15 | 2.00 |
| BSG | BSGE | |
|---|---|---|
| Carbohydrates (g/100 g dwb) | ||
| Total dietary fiber (TDF) | 49.66 ± 0.22 a | 48.47 ± 0.91 a |
| Insoluble dietary fiber (IDF) | 48.57 ± 0.15 b | 46.70± 0.63 a |
| Soluble dietary fiber (SDF) | 1.12 ± 0.03 a | 1.77 ± 0.00 b |
| Total starch | 4.498 ± 0.18 b | 4.14 ± 0.17 a |
| Digestible starch | 2.68± 0.11 b | 2.55 ± 0.06 a |
| Resistant starch | 1.71 ± 0.04 a | 1.69 ± 0.01 a |
| Free glucose | 0.13 ±0.00 a | 0.54 ± 0.02 b |
| Proteins (g/100 g dwb) | 29.78 ± 0.33 a | 29.46 ± 0.11 a |
| Lipids (g/100 g dwb) | 9.61 ± 0.04 a | 10.34 ± 0.01 b |
| Ash (g/100 g dwb) | 3.27 ± 0.02 a | 3.31 ± 0.06 a |
| Amino Acid (g/100 g dwb) | BSG | BSGE | |
|---|---|---|---|
| Nonessential amino acids (NEAA) | Alanine (Ala) | 2.054 ± 0.028 b | 1.888 ± 0.039 a |
| Arginine (Arg) | 0.996 ± 0.022 b | 0.857 ± 0.020 a | |
| Aspartic acid (Asp) | 1.954 ± 0.0246 b | 1.800 ± 0.033 a | |
| Cysteine (Cys) | 0.220 ± 0.017 b | 0.176 ± 0.006 a | |
| Glutamic acid (Glu) | 5.360 ± 0.226 a | 5.124 ± 0.107 a | |
| Glycine (Gly) | 1.075 ± 0.016 b | 0.988 ± 0.020 a | |
| Proline (Pro) | 2.994 ± 0.063 b | 2.729 ± 0.083 a | |
| Serine (Ser) | 1.460 ± 0.011 b | 1.361 ± 0.030 a | |
| Tyrosine (Tyr) | 0.939 ± 0.020 b | 0.768 ± 0.009 a | |
| Essential amino acids (EAA) | Histidine (His) | 0.832 ± 0.016 b | 0.755 ± 0.017 a |
| Isoleucine (Ile) | 1.108 ± 0.009 b | 1.023 ± 0.020 a | |
| Leucine (Leu) | 3.188 ± 0.150 a | 3.003 ± 0.042 a | |
| Lysine (Lys) | 0.931 ± 0.032 b | 0.857 ± 0.025 a | |
| Methionine (Met) | 0.589 ± 0.012 b | 0.527 ± 0.008 a | |
| Phenylalanine (Phe) | 1.593 ± 0.028 b | 1.472 ± 0.030 a | |
| Threonine (Thr) | 1.111 ± 0.011 b | 1.014 ± 0.019 a | |
| Tryptophan (Trp) | n.d. | n.d. | |
| Valine (Val) | 1.453 ± 0.019 b | 1.356 ± 0.018 a | |
| Total | 27.859 ± 0.264 b | 25.698 ± 0.419 a |
| BSG | BSGE | |
|---|---|---|
| EAA (g/100 g of protein) | 38.786 ± 0.272 a | 39.351 ± 0.188 b |
| Phe + Tyr (AAA) (g/100 g of protein) | 8.505 ± 0.151 b | 7.605 ± 0.084 a |
| Ile + Leu + Val (BCAA) (g/100 g of protein) | 19.310 ± 0.561 b | 18.272 ± 0.243 a |
| Fischer’s ratio (BCAA/AAA) | 2.271 ± 0.092 a | 2.403 ± 0.037 a |
| Lys/Arg ratio | 0.935 ± 0.045 a | 0.999 ± 0.026 a |
| %EAAI | 76.074 ± 0.261 b | 70.698 ± 1.1888 a |
| BV | 71.220 ± 0.285 b | 65.361 ± 1.296 a |
| NI | 22.647 ± 0.078 b | 21.047 ± 0.359 a |
| PER1 | 4.671 ± 0.222 a | 4.400 ± 0.073 a |
| PER2 | 4.724 ± 0.224 a | 4.434 ± 0.068 a |
| PER3 | 5.009 ± 0.443 a | 4.993 ± 0.098 a |
| SAT1 (g/100 g of protein) | 24.443 ± 0.375 b | 22.625 ± 0.326 a |
| SAT2 (g/100 g of protein) | 6.885 ± 0.102 b | 6.050 ± 0.071 a |
| Sample | Extraction Method | EPC (mg GAE/g dwb) | ABTS Method (µmol TE/g dwb) | ORAC Method (µmol TE/g dwb) |
|---|---|---|---|---|
| BSG | EHAA 5 min | 1.403 ± 0.056 b | 27.126 ± 1.287 a | 25.475 ± 1.305 a |
| EHAA 24 h | 1.569 ± 0.038 c | 38.548 ± 0.851 a,b | 50.188 ± 0.873 b | |
| Alkaline 5 min | 1.587 ± 0.056 c | 38.945 ± 0.651 c | 216.232 ± 7.951 c | |
| Alkaline 24 h | 3.623 ± 0.124 e | 79.143 ± 2.834 e | 304.923 ± 5.752 d | |
| BSGE | EHAA 5 min | 1.206 ± 0.044 a | 26.904 ± 0.906 a | 23.337 ± 1.395 a |
| EHAA 24 h | 1.623 ± 0.088 c | 32.084 ± 1.590 b | 67.591 ± 0.037 b | |
| Alkaline 5 min | 2.366 ± 0.110 d | 52.200 ± 1.215 d | 311.769 ± 6.654 d | |
| Alkaline 24 h | 4.221 ± 0.114 f | 92.955 ± 4.775 f | 492.470 ± 8.691 e |
| Proposed Compound | Molecular Formula | Molar Mass (g/mol) | Retention Time (min) | Relative Percentage in BSG * (%) | Relative Percentage in BSGE * (%) | Variation after Extrusion ** (%) |
|---|---|---|---|---|---|---|
| p-coumaric acid | C9H8O3 | 163.0 | 16.4 | 0.4 | 0.9 | 111.1 |
| 2-(3-hydroxyphenyl) propionic acid | C9H10O3 | 165.1 | 16.7 | 51.6 | 58.8 | 27.5 |
| Ferulic acid | C15H18O8 | 193.1 | 20.0 | 0.2 | 0.3 | 72.4 |
| p-coumaric acid glucoside | C15H18O8 | 325.1 | 18.2 | 1.3 | 1.5 | 23.9 |
| Hydrodiferulic | C20H18O8 | 385.1 | 6.7 | 7.5 | 6.2 | −7.5 |
| Dihydrocaffeic acid | C9H10O4 | 181.1 | 6.7 | 24.9 | 19.4 | −12.7 |
| Dihydrobenzoic acid | C7H6O4 | 153.0 | 4.3 | 4.0 | 3.0 | −15.6 |
| Hydroxybenzoic acid | C7H6O3 | 137.0 | 7.6 | 5.4 | 4.5 | −5.2 |
| Benzoic acid | C7H6O2 | 121.0 | 11.5 | 4.6 | 5.7 | 31.3 |
| BSG | BSGE | |
|---|---|---|
| Total aerobic count (cfu/g) | (5.150 ± 1.626) × 106 b | (6.550 ± 0.636) × 102 a |
| Total aerobic endospores count (cfu/g) | (3.250 ± 0.495) × 104 b | 6.000 ± 1.414 a |
| Total molds and yeasts count (cfu/g) | (1.700 ± 0.282) × 106 a | n.d. |
| Gluten (ppm) | (30.519 ± 2.361) × 105 b | (25.258 ± 1.413) × 105 a |
| Acrilamida | <20 µg/kg a | <20 µg/kg a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Barrutia, M.B.; del Castillo, M.D.; Arcia, P.; Cozzano, S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods 2022, 11, 1403. https://doi.org/10.3390/foods11101403
Gutiérrez-Barrutia MB, del Castillo MD, Arcia P, Cozzano S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods. 2022; 11(10):1403. https://doi.org/10.3390/foods11101403
Chicago/Turabian StyleGutiérrez-Barrutia, María Belén, María Dolores del Castillo, Patricia Arcia, and Sonia Cozzano. 2022. "Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet" Foods 11, no. 10: 1403. https://doi.org/10.3390/foods11101403
APA StyleGutiérrez-Barrutia, M. B., del Castillo, M. D., Arcia, P., & Cozzano, S. (2022). Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods, 11(10), 1403. https://doi.org/10.3390/foods11101403