Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Okra Pectin
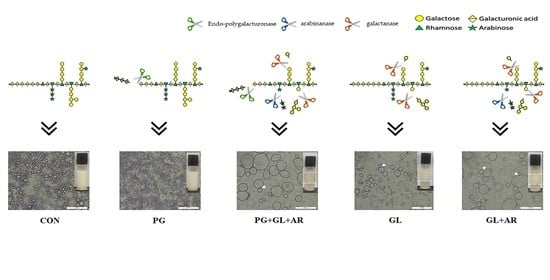
2.3. Enzymatic Modification of OKP
2.4. Physicochemical and Monosaccharide Analyses
2.4.1. Physicochemical Compositions
2.4.2. Monosaccharide Analysis
2.5. NMR and FT-IR Structural Characteristics
2.6. Molecular Weight and Apparent Viscosity Determination
2.7. Emulsion Preparation and Characteristics
2.8. Statistical Analysis
3. Results and Discussion
3.1. Enzymatic Treatment and Composition of Pectins
3.2. Monosaccharide and Molar Ratios
3.3. FT-IR and NMR Characteristics
3.4. Molecular and Rheological Properties
3.5. Emulsion Characteristics
3.6. Principal Component Analysis of Structure–Function Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705. [Google Scholar] [CrossRef]
- Kpodo, F.; Agbenorhevi, J.; Alba, K.; Oduro, I.; Morris, G.; Kontogiorgos, V. Structure-function relationships in pectin emulsification. Food Biophys. 2018, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Yang, Z.; Yang, J.; Liu, F.; Xu, X.; Pan, S. Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin. Foods 2021, 10, 2459. [Google Scholar] [CrossRef] [PubMed]
- Humerez-Flores, J.N.; Verkempinck, S.H.; De Bie, M.; Kyomugasho, C.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Understanding the impact of diverse structural properties of homogalacturonan rich citrus pectin-derived compounds on their emulsifying and emulsion stabilizing potential. Food Hydrocoll. 2022, 125, 107343. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455. [Google Scholar] [CrossRef]
- Mendez, D.A.; Fabra, M.J.; Martínez-Abad, A.; Martínez-Sanz, M.; Gorria, M.; López-Rubio, A. Understanding the different emulsification mechanisms of pectin: Comparison between watermelon rind and two commercial pectin sources. Food Hydrocoll. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohydr. Res. 2009, 344, 1842. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra extracts as emulsifiers for acidic emulsions. Food Res. Int. 2013, 54, 1730. [Google Scholar] [CrossRef]
- Xu, K.; Martinez, M.M.; Yang, B.; Guo, M. Fine structure, physicochemical and antioxidant properties of LM-pectins from okra pods dried under different techniques. Carbohydr. Polym. 2020, 241, 116272. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.; Panneerselvam, K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J. Pharm. Bioallied Sci. 2011, 3, 397. [Google Scholar] [PubMed]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Alba, K.; Sagis, L.; Kontogiorgos, V. Engineering of acidic O/W emulsions with pectin. Colloids Surf. B Biointerfaces 2016, 145, 301. [Google Scholar] [CrossRef]
- Schmidt, U.; Schmidt, K.; Kurz, T.; Endreß, H.-U.; Schuchmann, H. Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59. [Google Scholar] [CrossRef]
- Zhou, Y.; Mei, Y.; Luo, T.; Chen, W.; Zhong, Q.; Chen, H.; Chen, W. Study on the Relationship between Emulsion Properties and Interfacial Rheology of Sugar Beet Pectin Modified by Different Enzymes. Molecules 2021, 26, 2829. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.; Vaes, G.; Wouters, K.; Magnée, J.; Hendrickx, M.; Van Loey, A. Advanced insight into the emulsifying and emulsion stabilizing capacity of carrot pectin subdomains. Food Hydrocoll. 2020, 102, 105594. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, P.; Wang, W.; Wang, M. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: The case of okra polysaccharide. Food Hydrocoll. 2020, 102, 105586. [Google Scholar] [CrossRef]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, L.; Yagoub, A.E.A.; Yu, X.; Ma, H.; Zhou, C. Effects of ultrasound, freeze-thaw pretreatments and drying methods on structure and functional properties of pectin during the processing of okra. Food Hydrocoll. 2021, 120, 106965. [Google Scholar] [CrossRef]
- Kpodo, F.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.; Oduro, I.; Morris, G.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323. [Google Scholar] [CrossRef] [Green Version]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Hahn, D.; Lee, W.Y. Physicochemical and Functional Properties of Okra Leaf Polysaccharides Extracted at Different pHs. Chemistry 2022, 4, 405–418. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification—A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Verkempinck, S.H.; Kyomugasho, C.; Moldenaers, P.; Van Loey, A.M.; Hendrickx, M.E. Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties. Foods 2021, 10, 1586. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221. [Google Scholar] [CrossRef]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity relationship of Citrus segment membrane RG-I pectin against Galectin-3: The galactan is not the only important factor. Carbohydr. Polym. 2020, 245, 116526. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157. [Google Scholar] [CrossRef]
- Voragen, A.; Schols, H.; Pilnik, W. Determination of the degree of methylation and acetylation of pectins by HPLC. Food Hydrocoll. 1986, 1, 65. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.-W.; Zhu, S.; Yin, H.-P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef] [PubMed]
- Chantrapornchai, W.; Clydesdale, F.M.; McClements, D.J. Understanding Colors in Emulsions; ACS Publications: Washington, DC, USA, 2008. [Google Scholar]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yu, C.; Zaeim, D.; Wu, D.; Hu, X.; Ye, X.; Chen, S. Increasing RG-I content and lipase inhibitory activity of pectic polysaccharides extracted from goji berry and raspberry by high-pressure processing. Food Hydrocoll. 2022, 126, 107477. [Google Scholar] [CrossRef]
- Liang, W.-L.; Liao, J.-S.; Qi, J.-R.; Jiang, W.-X.; Yang, X.-Q. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef]
- Ai, C.; Meng, H.; Lin, J.; Tang, X.; Guo, X. Emulsification properties of alkaline soluble polysaccharide from sugar beet pulp: Effect of acetylation and methoxylation. Food Hydrocoll. 2022, 124, 107361. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105. [Google Scholar] [CrossRef] [PubMed]
- Bindereif, B.; Eichhöfer, H.; Bunzel, M.; Karbstein, H.; Wefers, D.; Van der Schaaf, U. Arabinan side-chains strongly affect the emulsifying properties of acid-extracted sugar beet pectins. Food Hydrocoll. 2021, 121, 106968. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chem. 2015, 176, 82. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Zheng, Y.; Zhang, H.; Chen, J.; Yan, L.; Ding, T.; Linhardt, R.J.; Orfila, C.; Liu, D. Fast preparation of rhamnogalacturonan I enriched low molecular weight pectic polysaccharide by ultrasonically accelerated metal-free Fenton reaction. Food Hydrocoll. 2019, 95, 551. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Techniques for the chemical and physicochemical characterization of polysaccharides. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–74. [Google Scholar]
- Nguyen, T.T.; Olawuyi, I.F.; Park, J.J.; Lee, W.Y. Characteristics of edible jelly enriched with antioxidant and calcium-rich fractions of dandelion leaf polysaccharide extracts. J. Food Meas. Charact. 2022, 16, 1312–1324. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F.; Harding, S.E. Physical characterisation of the rhamnogalacturonan and homogalacturonan fractions of sugar beet (Beta vulgaris) pectin. Carbohydr. Polym. 2010, 82, 1161. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473. [Google Scholar] [CrossRef]
- Liu, X.-X.; Yan, Y.-Y.; Liu, H.-M.; Wang, X.-D.; Qin, G.-Y. Emulsifying and structural properties of polysaccharides extracted from Chinese yam by an enzyme-assisted method. LWT 2019, 111, 242. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Aghlara, A.; Hamid, N.S.; Yusof, S.; Chern, B.H. Influence of pectin and CMC on physical stability, turbidity loss rate, cloudiness and flavor release of orange beverage emulsion during storage. Carbohydr. Polym. 2008, 73, 83. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, Z.; Cheng, Y.; Xu, F.; Cheng, X.; Wu, P. Physicochemical characterization and emulsifying properties evaluation of RG-I enriched pectic polysaccharides from Cerasus humilis. Carbohydr. Polym. 2021, 260, 117824. [Google Scholar] [CrossRef] [PubMed]
- Pi, F.; Liu, Z.; Guo, X.; Guo, X.; Meng, H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocoll. 2019, 89, 792. [Google Scholar] [CrossRef]
- Nakamura, A.; Maeda, H.; Corredig, M. Emulsifying properties of enzyme-digested soybean soluble polysaccharide. Food Hydrocoll. 2006, 20, 1029. [Google Scholar] [CrossRef]





| Treatment Code | Treatment Description |
|---|---|
| CON | Okra pectin (OKP) obtained by hot sodium acetate buffer (0.05 M, pH 4.5) extraction. |
| PG | OKP hydrolyzed with endo-polygalacturonanase (0.4 U/mg) enzyme |
| PG+GL+AR | OKP hydrolyzed with endo-polygalacturonanase (0.4 U/mg), endo-1,4-β-galactanase (0.4 U/mg), β-galactosidase (0.4 U/mg), endo-1,5-α-arabinanase (0.2 U/mg), and α-l-Arabinofuranosidase (0.2 U/mg) enzymes |
| GL | OKP hydrolyzed with endo-1,4-β-galactanase (0.4 U/mg) and β-galactosidase (0.4 U/mg) enzymes |
| GL+AR | OKP hydrolyzed with endo-1,4-β-galactanase (0.4 U/mg), β-galactosidase (0.4 U/mg), endo-1,5-α-arabinanase (0.2 U/mg), and α-l-arabinofuranosidase (0.2 U/mg) enzymes. |
| CON | PG | PG+GL+AR | GL | GL+AR | |
|---|---|---|---|---|---|
| Recovery 1 (%) | - | 80.26 ± 0.90 b | 71.20 ± 0.36 d | 82.36 ± 0.54 a | 78.09 ± 1.26 c |
| Total protein (%) | 1.56 ± 0.04 b | 1.53 ± 0.04 b | 1.66 ± 0.04 a | 1.57 ± 0.02 b | 1.60 ± 0.05 ab |
| Degree of acetylation 2 (%) | 43.23 ± 0.66 c | 47.45 ± 0.07 a | 46.61 ± 0.54 a | 44.83 ± 0.23 b | 37.70 ± 0.28 d |
| Degree of methylation 2 (%) | 14.59 ± 1.11 a | 8.13 ± 0.37 d | 6.05 ± 0.13 c | 10.96 ± 0.05 b | 7.89 ± 0.57 c |
| Zeta potential (mV) | −34.40 ± 1.86 bc | −39.60 ± 1.56 c | −25.54± 1.81 a | −31.42 ± 2.69 b | −21.15 ± 2.33 a |
| Monosaccharides 3 (g/100 g) | |||||
| Mannose | 0.32 ± 0.07 a (0.4) | - | - | - | - |
| Rhamnose | 13.44 ± 0.09 a (15.0) | 13.68 ± 0.26 a (18.4) | 12.35 ± 0.58 b (18.4) | 10.03 ± 0.53 d (16.0) | 11.26 ± 0.03 bc (16.2) |
| Galacturonic acid | 47.91 ± 1.08 a (53.6) | 35.85 ± 0.09 c (48.2) | 36.49 ± 0.27 c (54.3) | 37.03 ± 0.32 c (59.0) | 41.88 ± 2.12 b (60.2) |
| Glucose | 0.88 ± 0.01 a (1.0) | 0.69 ± 0.07 b (0.9) | 0.44 ± 0.02 c (0.7) | 0.34 ± 0.05 d (0.5) | 0.47 ± 0.02 c (0.7) |
| Galactose | 25.66 ± 0.28 a (28.7) | 22.93 ± 0.23 b (30.8) | 17.49 ± 0.18 c (26.0) | 14.40 ± 0.25 e (23.0) | 15.63 ± 0.30 d (22.5) |
| Arabinose | 1.17 ± 0.03 b (1.3) | 1.22 ± 0.02 a (1.7) | 0.40 ± 0.03 c (0.6) | 0.97 ± 0.04 b (1.6) | 0.37 ± 0.00 c (0.5) |
| Molecular parameters | |||||
| Number Average Mn (kDa) | 471.02 ± 12.13 a | 178.17 ± 0.82 d | 210.48 ± 7.57 c | 215.61 ± 1.12 b | 156.57 ± 2.27 e |
| Number Average Mw (kDa) | 1868.90 ± 18.80 a | 1209.39 ± 25.22 c | 1091.54 ± 3.18 d | 1276.83 ± 17.78 b | 1045.39 ± 7.56 e |
| Polydispersity (Mw/Mn) | 3.97 | 6.79 | 5.19 | 5.92 | 6.68 |
| Molar Ratios 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| MR1 | MR2 | MR3 | MR4 | MR5 | HG | RG-I | HG/RG-I | |
| CON | 0.28 | 0.44 | 0.42 | 0.02 | 1.19 | 38.56 | 60.10 | 0.64 |
| PG | 0.38 | 0.49 | 0.46 | 0.02 | 0.95 | 29.80 | 69.27 | 0.43 |
| PG+GL+AR | 0.34 | 0.37 | 0.36 | 0.01 | 1.21 | 35.93 | 63.41 | 0.57 |
| GL | 0.27 | 0.33 | 0.31 | 0.02 | 1.46 | 43.02 | 56.44 | 0.76 |
| GL+AR | 0.27 | 0.30 | 0.29 | 0.01 | 1.54 | 43.96 | 55.37 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olawuyi, I.F.; Park, J.J.; Park, G.D.; Lee, W.Y. Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin. Foods 2022, 11, 1497. https://doi.org/10.3390/foods11101497
Olawuyi IF, Park JJ, Park GD, Lee WY. Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin. Foods. 2022; 11(10):1497. https://doi.org/10.3390/foods11101497
Chicago/Turabian StyleOlawuyi, Ibukunoluwa Fola, Jong Jin Park, Gwang Deok Park, and Won Young Lee. 2022. "Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin" Foods 11, no. 10: 1497. https://doi.org/10.3390/foods11101497
APA StyleOlawuyi, I. F., Park, J. J., Park, G. D., & Lee, W. Y. (2022). Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin. Foods, 11(10), 1497. https://doi.org/10.3390/foods11101497