Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice
Abstract
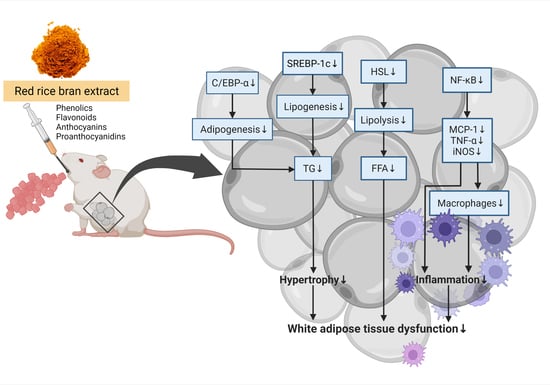
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ethanol Extracts from Red Rice Bran Samples
2.2. Analysis of Bioactive Compounds in RRBE
2.3. Animal Study
2.4. Histological Analysis
2.5. Determination of Lipids in eWATs
2.6. Gene Expression Analysis
2.7. Serum Analysis
2.8. Statistical Analysis
3. Results
3.1. Extraction Yield and Bioactive Components of RRBE
3.2. Effects of RRBE on Parameters Related to the HFD-Induced Obesity Mouse Model
3.3. Effect of RRBE on the Histological Appearance of the Adipose Tissue in Obese Mice
3.4. Effects of RRBE on Adipose Tissue Lipid Content and Adipogenesis- and Lipid Metabolism-Related Gene Expression in Obese Mice
3.5. Effects of RRBE on Adipose Tissue Inflammation-Related Gene Expression and Serum Inflammatory Mediators in Obese Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Piché, M.E.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: An update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Song, J.; Mao, X. Milk Fat Globule Membrane attenuates high-fat diet-induced obesity by inhibiting adipogenesis and increasing uncoupling protein 1 expression in white adipose tissue of mice. Nutrients 2018, 10, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, Y.; Ma, S.R.; Zuo, Z.Y.; Wu, Y.B.; Kong, W.J.; Wang, A.P.; Jiang, J.D. Berberine inhibits adipocyte differentiation, proliferation and adiposity through down-regulating galectin-3. Sci. Rep. 2019, 9, 13415. [Google Scholar] [CrossRef] [Green Version]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Y.; Shu, T.; Wang, J. Cytokines and inflammation in adipogenesis: An updated review. Front. Med. 2019, 13, 314–329. [Google Scholar] [CrossRef]
- Corrêa, L.H.; Corrêa, R.; Farinasso, C.M.; de Sant'Ana Dourado, L.P.; Magalhães, K.G. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: New implications in cancer progression. Front. Immunol. 2017, 8, 1129. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Rong, X.; Zheng, C.; Guo, J. Anti-Lipolysis Induced by Insulin in Diverse Pathophysiologic Conditions of Adipose Tissue. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1575–1585. [Google Scholar] [CrossRef]
- Surarit, W.; Jansom, C.; Lerdvuthisopon, N.; Kongkham, S.; Hansakul, P. Evaluation of antioxidant activities and phenolic subtype contents of ethanolic bran extracts of Thai pigmented rice varieties through chemical and cellular assays. Int. J. Food Sci. Technol. 2015, 50, 990–998. [Google Scholar] [CrossRef]
- Boue, S.M.; Daigle, K.W.; Chen, M.H.; Cao, H.; Heiman, M.L. Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J. Agric. Food Chem. 2016, 64, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.W.; Kobayashi, K.; Shen, L.; Inagaki, J.; Ide, M.; Hwang, S.S.; Matsuura, E. Antioxidative attributes of rice bran extracts in ameliorative effects of atherosclerosis-associated risk factors. Heliyon 2020, 6, e05743. [Google Scholar] [CrossRef] [PubMed]
- Tantipaiboonwong, P.; Pintha, K.; Chaiwangyen, W.; Chewonarin, T.; Pangjit, K.; Chumphukam, O.; Kangwan, N.; Suttajit, M. Anti-hyperglycaemic and anti-hyperlipidaemic effects of black and red rice in streptozotocin-induced diabetic rats. ScienceAsia 2017, 43, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Hussein, E.; Abdel-Megeid, N.S.; Melebary, S.J.; AL-Harbi, M.S.; Saleh, A.A. Growth performance, antioxidant activity, immune status, meat quality, liver fat content, and liver histomorphology of broiler chickens fed rice bran oil. Animals 2021, 11, 3410. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Kongthitilerd, P.; Suantawee, T.; Cheng, H.; Thilavech, T.; Marnpae, M.; Adisakwattana, S. Anthocyanin-enriched Riceberry rice extract inhibits cell proliferation and adipogenesis in 3T3-L1 preadipocytes by downregulating adipogenic transcription factors and their targeting genes. Nutrients 2020, 12, 2480. [Google Scholar] [CrossRef]
- Liu, D.; Ji, Y.; Zhao, J.; Wang, H.; Guo, Y.; Wang, H. Black rice (Oryza sativa L.) reduces obesity and improves lipid metabolism in C57BL/6J mice fed a high-fat diet. J. Funct. Foods. 2020, 64, 103605. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet- induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019. [Google Scholar] [CrossRef]
- Hwang, I.; Jo, K.; Shin, K.C.; Kim, J.I.; Ji, Y.; Park, Y.J.; Park, J.; Jeon, Y.G.; Ka, S.; Suk, S.; et al. GABA-stimulated adipose-derived stem cells suppress subcutaneous adipose inflammation in obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 11936–11945. [Google Scholar] [CrossRef] [Green Version]
- Gea-Sorlí, S.; Bonjoch, L.; Closa, D. Differences in the inflammatory response induced by acute pancreatitis in different white adipose tissue sites in the rat. PLoS ONE 2012, 7, e41933. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naowaboot, J.; Somparn, N.; Saentaweesuk, S.; Pannangpetch, P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phytother. Res. 2015, 29, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Xu, X.; Wang, J.; Li, X.; Meng, K.; Yang, P. Serum metabolomics analysis for biomarkers of Lactobacillus plantarum FRT4 in high-fat diet-induced obese mice. Foods 2022, 11, 184. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Park, Y.; Jang, J.; Lee, D.; Yoon, M. Vitamin C inhibits visceral adipocyte hypertrophy and lowers blood glucose levels in high-fat-diet-induced obese C57BL/6J mice. Biomed. Sci. Lett. 2018, 24, 311–318. [Google Scholar] [CrossRef]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae Pulliat is associated with the activation of AMPK signaling pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Jia, Y.; Du, X.; Wang, Y.; Yang, Z.; Li, K. Study of physicochemical stability of anthocyanin extracts from black peanut skin and their digestion enzyme and adipogenesis inhibitory activities. LWT 2019, 107, 107–116. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, Q.; Tong, X.; Liu, L.; Dong, L.; Huang, F.; Deng, Y.; Jia, X.; Chi, J.; Zhang, M. Rice bran phenolic extractsupplementation ameliorates impaired lipid metabolism in high-fat-diet fed mice through AMPK activation in liver. J. Funct. Foods 2020, 73, 104131. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lai, H.M. Bioactive compounds and antioxidative activity of colored rice bran. J. Food Drug Anal. 2016, 24, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilavenil, S.; Kim, D.H.; Srigopalram, S.; Kuppusamy, P.; Arasu, M.V.; Lee, K.D.; Lee, J.C.; Song, Y.H.; Jeong, Y.-I.; Choi, K.C. Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods 2017, 37, 293–302. [Google Scholar] [CrossRef]
- Rivera-Piza, A.; An, Y.J.; Kim, D.K.; Lee, S.H.; Kim, J.B.; Choi, J.S.; Lee, S.J. Protocatechuic acid enhances osteogenesis, but inhibits adipogenesis in C3H10T1/2 and 3T3-L1 cells. J. Med. Food 2017, 20, 309–319. [Google Scholar] [CrossRef]
- Minatel, I.O.; Lee, Y.M.; Yoon, H.; Yoon, Y.; Han, S.I.; Correa, C.R.; Fecchio, D.; Yeum, K.J. Antiadipogenic activity of γ-oryzanol and its stability in pigmented rice. J. Med. Food. 2016, 19, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Xiong, Z.; Yu, S.; Zhou, B.; Ling, Y.; Zheng, Z.; Shi, G.; Wu, Y.; Qian, X. Ginsenoside Rb1 inhibits free fatty acids-induced oxidative stress and inflammation in 3T3-L1 adipocytes. Mol. Med. Rep. 2017, 16, 9165–9172. [Google Scholar] [CrossRef] [Green Version]
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Campos, C.; Rodriguez, A.; Vargas, R.; Magrone, T.; Videla, L.A. Protective effects of eicosapentaenoic acid plus hydroxytyrosol supplementation against white adipose tissue abnormalities in mice fed a high-fat diet. Molecules 2020, 25, 4433. [Google Scholar] [CrossRef]
- Naowaboot, J.; Nanna, U.; Chularojmontri, L.; Songtavisin, T.; Tingpej, P.; Sattaponpan, C.; Jansom, C.; Wattanapitayakul, S. Mentha cordifolia leaf extract improves hepatic glucose and lipid metabolism in obese mice fed with high-fat diet. Prev. Nutr. Food Sci. 2021, 26, 157–165. [Google Scholar] [CrossRef]
- Naowaboot, J.; Nanna, U.; Chularojmontri, L.; Tingpej, P.; Pannangpetch, P. Effect of Thunbergia laurifolia water extracts on hepatic insulin resistance in high-fat diet-induced obese mice. Asian Pac. J. Trop. Biomed. 2021, 11, 97–104. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin–TLR4–NF-κB pathway. J. Agric. Food Chem. 2020, 68, 1237–1247. [Google Scholar] [CrossRef]
- Zhang, Q.; de Mejia, E.G. Protocatechuic acid attenuates adipogenesis-induced inflammation and mitochondrial dysfunction in 3T3-L1 adipocytes by regulation of AMPK pathway. J. Funct. Foods. 2020, 69, 103972. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]




| Bioactive Compounds | Content |
|---|---|
| Phenolics (mg GAE/g) | 326.85 ± 7.52 |
| Flavonoids (mg CE/g) | 82.84 ± 5.44 |
| Anthocyanins (μg C-3-GE/g) | 24.20 ± 13.70 |
| Proanthocyanidins (mg CE/g) | 71.49 ± 5.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munkong, N.; Lonan, P.; Mueangchang, W.; Yadyookai, N.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice. Foods 2022, 11, 1865. https://doi.org/10.3390/foods11131865
Munkong N, Lonan P, Mueangchang W, Yadyookai N, Kanjoo V, Yoysungnoen B. Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice. Foods. 2022; 11(13):1865. https://doi.org/10.3390/foods11131865
Chicago/Turabian StyleMunkong, Narongsuk, Piyanuch Lonan, Wirinya Mueangchang, Narissara Yadyookai, Vaiphot Kanjoo, and Bhornprom Yoysungnoen. 2022. "Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice" Foods 11, no. 13: 1865. https://doi.org/10.3390/foods11131865
APA StyleMunkong, N., Lonan, P., Mueangchang, W., Yadyookai, N., Kanjoo, V., & Yoysungnoen, B. (2022). Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice. Foods, 11(13), 1865. https://doi.org/10.3390/foods11131865