Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet
Abstract
:1. Introduction
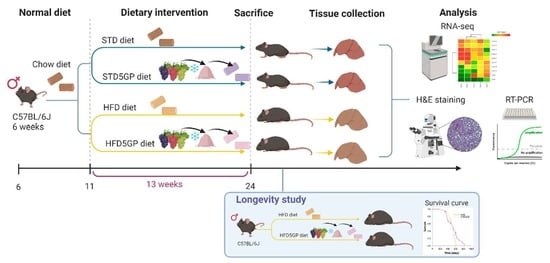
2. Materials and Methods
2.1. Animals and Diets
2.1.1. Semi-Synthetic Diets
2.1.2. Survival Analyses
2.1.3. Liver Analyses
2.2. Liver Collection
2.3. Histopathological Examination of Liver
2.4. RNA-Sequencing
2.4.1. Differential Expression Analyses
2.4.2. Gene Ontology Analyses
2.4.3. KEGG and Reactome Pathway Analyses
2.5. RT-qPCR
2.6. Statistical Analyses
3. Results
3.1. Effect of Diets on Mouse Body Weight
3.2. Grape Powder Supplementation of High-Fat Diet Enhances Mouse Longevity
3.3. Grape Powder Supplementation of High-Fat Diet Reduces Lipid Accumulation in Mouse Liver
3.4. Dietary Fat Content and Supplementation with Grape Powder Alters Gene Expression Profiles in Mouse Liver
3.5. High Dietary Fat Content Alters Gene Expression in Mouse Liver
3.6. Grape Powder Supplementation Alters Gene Expression in Mouse Liver
3.7. Grape Powder Supplementation of High Fat Diet Alters Genes Responsible for Lipid Metabolism in Mouse Liver
3.8. Grape Powder Supplementation of Standard but Not High-Fat Diet Enhances Antioxidant Potential in Mouse Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Doll, R. An epidemiological perspective of the biology of cancer. Cancer Res. 1978, 38, 3573–3583. [Google Scholar]
- Phillips, R.L. Role of life-style and dietary habits in risk of cancer among Seventh-day Adventists. Cancer Res. 1975, 35, 3513–3522. [Google Scholar]
- Buell, P.; Dunn, J.E. Cancer mortality among Japanese issei and nisei of California. Cancer 1965, 18, 656–664. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 14 January 2022).
- Pallazola, V.A.; Davis, D.M.; Whelton, S.P.; Cardoso, R.; Latina, J.M.; Michos, E.D.; Sarkar, S.; Blumenthal, R.S.; Arnett, D.K.; Stone, N.J.; et al. A Clinician’s Guide to Healthy Eating for Cardiovascular Disease Prevention. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Owuor, E.D.; Kong, A.-N.T. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A Historical Overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Puchau, B.; Martínez, J.A. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: A translational study. Nutr. Metab. 2010, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Pezzuto, J.M.; Vang, O. Perspective: A Positive Cocktail Effect of the Bioactive Components in the Diet. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Springer: Berlin/Heidelberg, Germany, 2020; pp. 613–629. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Xiao, Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med. Hypotheses 2006, 67, 833–838. [Google Scholar] [CrossRef]
- Shin, M.-O.; Moon, J.-O. Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethylnitrosamine in rats. Nutr. Res. Pract. 2010, 4, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Crescenti, A.; del Bas, J.M.; Arola-Arnal, A.; Oms-Oliu, G.; Arola, L.; Caimari, A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J. Nutr. Biochem. 2015, 26, 912–920. [Google Scholar] [CrossRef]
- Lefevre, M.; Wiles, J.E.; Zhang, X.; Howard, L.R.; Gupta, S.; Smith, A.A.; Ju, Z.Y.; Delany, J.P. Gene expression microarray analysis of the effects of grape anthocyanins in mice: A test of a hypothesis-generating paradigm. Metabolism 2008, 57, S52–S57. [Google Scholar] [CrossRef] [Green Version]
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Grapes and Health; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A healthy approach to dietary fats: Understanding the science and taking action to reduce consumer confusion. Nutr. J. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Saliba, L.J.; Maffett, S. Hypertensive Heart Disease and Obesity: A Review. Heart. Fail. Clin. 2019, 15, 509–517. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, F.; Luo, Y.X.; Hu, P.; Ren, H.; Peng, M.L. The role of cytochrome P450 in nonalcoholic fatty liver induced by high-fat diet: A gene expression profile analysis. Zhonghua Gan Zang Bing Za Zhi Zhonghua Ganzangbing Zazhi Chin. J. Hepatol. 2017, 25, 285–290. [Google Scholar]
- Yang, D.; Jiang, H.; Lu, J.; Lv, Y.; Baiyun, R.; Li, S.; Liu, B.; Lv, Z.; Zhang, Z. Dietary grape seed proanthocyanidin extract regulates metabolic disturbance in rat liver exposed to lead associated with PPARα signaling pathway. Environ. Pollut. 2018, 237, 377–387. [Google Scholar] [CrossRef]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.-C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef] [Green Version]
- List, E.O.; Berryman, D.E.; Wright-Piekarski, J.; Jara, A.; Funk, K.; Kopchick, J.J. The effects of weight cycling on lifespan in male C57BL/6J mice. Int. J. Obes. 2013, 37, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- van Breemen, R.B.; Wright, B.; Li, Y.; Nosal, D.; Burton, T. Standardized Grape Powder for Basic and Clinical Research. In Grapes and Health; Pezzuto, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–26. [Google Scholar] [CrossRef]
- Joshi, T.; Patel, I.; Kumar, A.; Donovan, V.; Levenson, A.S. Grape Powder Supplementation Attenuates Prostate Neoplasia Associated with Pten Haploinsufficiency in Mice Fed High-Fat Diet. Mol. Nutr. Food Res. 2020, 64, e2000326. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bernstein, L.; Anderson, J.; Pike, M.C. Estimation of the Proportional Hazard in Two-Treatment-Group Clinical Trials. Biometrics 1981, 37, 513. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Doi, K.; Nakayama, H.; Uetsuka, K. The effect of fasting on hepatic lipid accumulation and transcriptional regulation of lipid metabolism differs between C57BL/6J and BALB/cA mice fed a high-fat diet. Toxicol. Pathol. 2008, 36, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Huang, H.; McIntosh, A.L.; Martin, G.G.; Landrock, D.; Chung, S.; Landrock, K.K.; Dangott, L.J.; Li, S.; Kier, A.B.; Schroeder, F. FABP1: A Novel Hepatic Endocannabinoid and Cannabinoid Binding Protein. Biochemistry 2016, 55, 5243–5255. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mohsen, A.-W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for Physical Association of Mitochondrial Fatty Acid Oxidation and Oxidative Phosphorylation Complexes. J. Biol. Chem. 2010, 285, 29834–29841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miinalainen, I.J.; Schmitz, W.; Huotari, A.; Autio, K.J.; Soininen, R.; van Themaat, E.V.L.; Baes, M.; Herzig, K.-H.; Conzelmann, E.; Hiltunen, J.K. Mitochondrial 2,4-dienoyl-CoA reductase deficiency in mice results in severe hypoglycemia with stress intolerance and unimpaired ketogenesis. PLoS Genet. 2009, 5, e1000543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konno, H.; Matin, A.; Maruo, Y.; Nakamura, S.; Baba, S. Liposomal ATP protects the liver from injury during shock. Eur. Surg. Res. 1996, 28, 140–145. [Google Scholar] [CrossRef]
- Liss, K.H.H.; Lutkewitte, A.J.; Pietka, T.; Finck, B.N.; Franczyk, M.; Yoshino, J.; Klein, S.; Hall, A.M. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J. Lipid Res. 2018, 59, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- van Kuijk, F.J.; Thomas, D.W.; Konopelski, J.P.; Dratz, E.A. Transesterification of phospholipids or triglycerides to fatty acid benzyl esters with simultaneous methylation of free fatty acids for gas-liquid chromatographic analysis. J. Lipid Res. 1986, 27, 452–456. [Google Scholar] [CrossRef]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Mol. Cell 2019, 77, 810–824. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta. 2013, 1831, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, L.; Chi, W.; Qiao, Y.; Zhang, J.; Song, X.; Liu, Y.; Li, L.; Jia, J.; Pilo, M.G.; Wang, J.; et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut 2020, 69, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, G.; Yin, J.; Li, L.; Tan, Y.; Wei, H.; Liu, B.; Deng, L.; Tang, J.; Chen, Y.; et al. GSTP1 and cancer: Expression, methylation, polymorphisms and signaling (Review). Int. J. Oncol. 2020, 56, 867–878. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2011, 821, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Guo, P.; Yu, H.; Wang, Y.; Chen, G. Ribosomal proteins: Insight into molecular roles and functions in hepatocellular carcinoma. Oncogene 2018, 37, 277–285. [Google Scholar] [CrossRef]
- Orsolic, I.; Jurada, D.; Pullen, N.; Oren, M.; Eliopoulos, A.G.; Volarevic, S. The relationship between the nucleolus and cancer: Current evidence and emerging paradigms. Semin. Cancer Biol. 2016, 37, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Draptchinskaia, N.; Gustavsson, P.; Andersson, B.; Pettersson, M.; Willig, T.-N.; Dianzani, I.; Ball, S.; Tchernia, G.; Klar, J.; Matsson, H.; et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet. 1999, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv. Cancer Res. 1989, 52, 205–255. [Google Scholar] [CrossRef]
- Soltis, A.R.; Kennedy, N.J.; Xin, X.; Zhou, F.; Ficarro, S.B.; Yap, Y.S.; Matthews, B.J.; Lauffenburger, D.A.; White, F.M.; Marto, J.A.; et al. Hepatic dysfunction caused by consumption of a high-fat diet. Cell Rep. 2017, 21, 3317–3328. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, J.E.; Fischel-Ghodsian, N.; Mougey, E.B.; O’Brien, T.W. Mitochondrial ribosomal proteins: Candidate genes for mitochondrial disease. Genet. Med. 2004, 6, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Krahmer, N.; Farese, R.V., Jr.; Walther, T.C. Balancing the fat: Lipid droplets and human disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Parande, F.; Dave, A.; Park, E.-J.; McAllister, C.; Pezzuto, J.M. Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes. Antioxidants 2022, 11, 414. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef]
- Kim, D.-G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.-A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-A.; Oh, C.H.; Kim, J.W.; Jeong, S.J.; Oh, I.-H.; Lee, J.S.; Park, K.-C.; Shim, J.-J. Association between non-alcoholic fatty liver disease and the risk of dementia: A nationwide cohort study. Liver Int. 2022, 42, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.; O’Donnell, A.; Zelber-Sagi, S.; Beiser, A.S.; Seshadri, S. Non-alcoholic fatty liver disease, liver fibrosis and patterns of regional amyloid and tau pathology in middle-aged adults: The Framingham Study. Alzheimer’s Dement. 2021, 17, e057808. [Google Scholar] [CrossRef]
- Lavie, C.J.; Loberg, K. The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier; Hudson Street Press: New York, NY, USA, 2014. [Google Scholar]
- Zheng, H.; Echave, P.; Mehta, N.; Myrskylä, M. Life-long body mass index trajectories and mortality in two generations. Ann. Epidemiol. 2021, 56, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, L.P.-C.; Andrade, G.M.; Mayoral, E.P.-C.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Blanton, W.P.; Belkina, A.; Lebrasseur, N.K.; Denis, G.V. Brd2 disruption in mice causes severe obesity without Type 2 diabetes. Biochem. J. 2009, 425, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Deeney, J.T.; Denis, G.V. Brd2 gene disruption causes ‘metabolically healthy’ obesity: Epigenetic and chromatin-based mechanisms that uncouple obesity from Type 2 diabetes. Vitam. Horm. 2013, 91, 49–75. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Stewart, W.C.L.; Burd, C.E.; Hester, M.E.; Greenberg, D.A. Brd2 haploinsufficiency extends lifespan and healthspan in C57B6/J mice. PLoS ONE 2020, 15, e0234910. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Brandt, J. The Grape Cure; Harmony Centre, Inc.: New York, NY, USA, 1928. [Google Scholar]
- Beyoğlu, D.; Park, E.-J.; Quiñones-Lombraña, A.; Dave, A.; Parande, F.; Pezzuto, J.M.; Idle, J.R. Addition of grapes to both a standard and a high-fat Western Pattern Diet modifies hepatic and urinary metabolite profiles in themouse. Food Funct. 2022, submitted.










| Standard Diet (TD.160157) 3 | Standard Diet with 5% Grape Powder (TD.160158) 3 | High-Fat Diet (TD.160154) 4 | High-Fat Diet with 5% Grape Powder (TD.160155) 4 | |
|---|---|---|---|---|
| Formula (g/kg) | ||||
| Casein | 195 | 195 | 195 | 195 |
| DL-Methionine | 3 | 3 | 3 | 3 |
| Sucrose | 191.1 | 191.1 | 191.1 | 191.1 |
| Dextrose, anhydrous | 66.45 | 44.3 | 64.45 | 44.3 |
| Fructose | 66.45 | 44.3 | 64.45 | 44.3 |
| Corn Starch | 235.03 | 232.88 | 167.43 | 161.37 |
| Maltodextrin | 100 | 100 | 0 | 0 |
| Anhydrous Milkfat 1 | 30 | 29.85 | 210 | 210 |
| Soybean oil | 10 | 10 | 0 | 0 |
| Cholesterol | 0 | 0 | 1.5 | 1.5 |
| Cellulose | 50 | 50 | 50 | 50 |
| Mineral Mix, AIN-76 (170,915) | 35 | 35 | 35 | 35 |
| Potassium Citrate, monohydrate | 4.03 | 2.69 | 4.03 | 2.69 |
| Calcium Carbonate | 4 | 4 | 4 | 4 |
| Vitamin Mix, Teklad (40,060) | 10 | 10 | 10 | 10 |
| Ethoxyquin, antioxidant | 0.04 | 0.04 | 0.04 | 0.04 |
| Grape powder, freeze-dried 2 | 0 | 50 | 0 | 50 |
| Diet Groups | Average (g) | Median (g) |
|---|---|---|
| STD | 24.13 ± 1.67 a | 23.58 |
| STD5GP | 22.93 ± 2.30 a | 23.36 |
| HFD | 33.23 ± 4.48 b | 33.27 |
| HFD5GP | 32.07 ± 5.79 b | 30.91 |
| Genes | HFD5GP vs. HFD Log2(FC) 1 | HFD5GP vs. HFD Padj Value | HFD vs. STD Log2(FC) 1 | HFD vs. STD Padj Value | Function |
|---|---|---|---|---|---|
| Fabp1 | 1.208 | 0.0001 | 0.421 | 0.006 | Transportation of FFA for degradation |
| Acads | 1.091 | <0.0001 | 0.239 | 0.043 | Mitochondrial degradation |
| Atp5j | 1.320 | <0.0001 | 0.294 | 0.027 | Mitochondrial degradation |
| Atp5j2 | 1.298 | <0.0001 | 0.311 | 0.053 | Mitochondrial degradation |
| Atp5k | 1.223 | <0.0001 | 0.461 | 0.012 | Mitochondrial degradation |
| Atp5l | 2.558 | <0.0001 | 0.458 | 0.030 | Mitochondrial degradation |
| Mogat1 | 1.198 | 0.013 | 1.149 | <0.0001 | Esterification |
| Plin5 | 1.864 | <0.0001 | 0.065 | 0.743 | Sequestration |
| Plin3 | 1.026 | <0.0001 | 0.083 | 0.870 | Sequestration |
| Abhd16a | 1.880 | <0.0001 | 0.261 | 0.090 | Hydrolysis |
| Abhd17b | 1.094 | 0.0005 | 0.186 | 0.419 | Hydrolysis |
| Plin4 | −0.941 | 0.010 | 1.861 | <0.0001 | Associate preferentially with small lipid droplets |
| Acaa1b | −1.440 | 0.0001 | 0.510 | 0.0001 | Cholesterol synthesis |
| Slc27a1 | −0.981 | 0.0003 | 0.645 | 0.0002 | Redistribution of lipids from fat and muscle to liver |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, A.; Park, E.-J.; Kumar, A.; Parande, F.; Beyoğlu, D.; Idle, J.R.; Pezzuto, J.M. Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet. Foods 2022, 11, 1984. https://doi.org/10.3390/foods11131984
Dave A, Park E-J, Kumar A, Parande F, Beyoğlu D, Idle JR, Pezzuto JM. Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet. Foods. 2022; 11(13):1984. https://doi.org/10.3390/foods11131984
Chicago/Turabian StyleDave, Asim, Eun-Jung Park, Avinash Kumar, Falguni Parande, Diren Beyoğlu, Jeffrey R. Idle, and John M. Pezzuto. 2022. "Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet" Foods 11, no. 13: 1984. https://doi.org/10.3390/foods11131984
APA StyleDave, A., Park, E. -J., Kumar, A., Parande, F., Beyoğlu, D., Idle, J. R., & Pezzuto, J. M. (2022). Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet. Foods, 11(13), 1984. https://doi.org/10.3390/foods11131984