Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Model Red Wine and Solvent Systems
2.3. HPLC Analysis
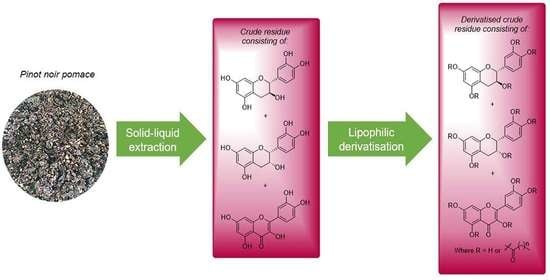
2.4. Materials and Solid–Liquid Extraction Protocol
2.5. Derivatization of the Flavonoid-Enriched Extract and Infrared Analysis
2.6. Antiproliferative Activity Procedures
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Solid–Liquid Extraction Method (Small-Scale Extraction Studies)
3.2. Large-Scale Solid–Liquid Extraction of Pinot noir Pomace
3.3. Fatty Acyl Derivatization of the Extract
3.4. Antiproliferative Activity Studies of the Products
3.5. Limitations to the Methods and Suggested Improvements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine State of the World Vitivinicultural Sector in 2020. Available online: http://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 3 June 2022).
- Mendes, J.A.S.; Xavier, A.M.R.B.; Evtuguin, D.V.; Lopes, L.P.C. Integrated Utilization of Grape Skins from White Grape Pomaces. Ind. Crops Prod. 2013, 49, 286–291. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity–a Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of Winery Waste vs. the Costs of Not Recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Dwyer, K.; Hossenian, F.; Pod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From Winery Waste to Bioactive Compounds and New Polymeric Biocomposites: A Contribution to the Circular Economy Concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of Yoghurts with Grape (Vitis vinifera) Seed Extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Rivera, O.M.P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic Acid and Biosurfactants Production from Hydrolyzed Distilled Grape Marc. Process Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of Grape Antioxidant Dietary Fibre on the Prevention of Lipid Oxidation in Minced Fish: Evaluation by Different Methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Jelley, R.E.; Deed, R.C.; Barker, D.; Parish-Virtue, K.; Fedrizzi, B. Fermentation of Sauvignon Blanc Grape Marc Extract Yields Important Wine Aroma 3-Sulfanylhexan-1-Ol (3SH). LWT 2020, 131, 109653. [Google Scholar] [CrossRef]
- Jelley, R.E.; Lee, A.J.; Zujovic, Z.; Villas-Boas, S.G.; Barker, D.; Fedrizzi, B. First Use of Grape Waste-Derived Building Blocks to Yield Antimicrobial Materials. Food Chem. 2022, 370, 131025. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. 11-Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–292. ISBN 978-0-12-809870-7. [Google Scholar]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [Green Version]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of Polyphenolic Content, Antioxidant Activity, Protection against ROS-Induced DNA Damage and Anticancer Activity of Vitis Vinifera Stem Extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Choleva, M.; Tsota, M.; Boulougouri, V.; Panara, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Anti-Platelet and Anti-Inflammatory Properties of an Ethanol-Water Red Grape Pomace Extract. Proc. Nutr. Soc. 2020, 79, E370. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and Cytotoxic Effects of Grape Pomace and Grape Seed Extracts on Colorectal Cancer Cell Lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic Compounds and Biopotential of Grape Pomace Extracts from Prokupac Red Grape Variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Olivero-David, R.; Ruiz-Roso, M.B.; Caporaso, N.; Perez-Olleros, L.; De las Heras, N.; Lahera, V.; Ruiz-Roso, B. In Vivo Bioavailability of Polyphenols from Grape By-Product Extracts, and Effect on Lipemia of Normocholesterolemic Wistar Rats. J. Sci. Food Agric. 2018, 98, 5581–5590. [Google Scholar] [CrossRef] [PubMed]
- Hu, M. Commentary: Bioavailability of Flavonoids and Polyphenols: Call to Arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Jelley, R.E.; Herbst-Johnstone, M.; Klaere, S.; Pilkington, L.I.; Grose, C.; Martin, D.; Barker, D.; Fedrizzi, B. Optimization of Ecofriendly Extraction of Bioactive Monomeric Phenolics and Useful Flavor Precursors from Grape Waste. ACS Sustain. Chem. Eng. 2016, 4, 5060–5067. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Synthesis, Antiproliferative Activity and Radical Scavenging Ability of 5-O-Acyl Derivatives of Quercetin. Molecules 2021, 26, 1608. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of Mono-Acylated Luteolin Derivatives, Evaluation of Their Antiproliferative and Radical Scavenging Activities and Implications on Their Oral Bioavailability. Sci. Rep. 2021, 11, 12595. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. Correlation of Wine Phenolic Composition versus Cyclic Voltammetry Response. Am. J. Enol. Vitic. 2002, 53, 294–302. [Google Scholar]
- Leung, E.Y.; Askarian-Amiri, M.E.; Singleton, D.C.; Ferraro-Peyret, C.; Joseph, W.R.; Finlay, G.J.; Broom, R.J.; Kakadia, P.M.; Bohlander, S.K.; Marshall, E.; et al. Derivation of Breast Cancer Cell Lines Under Physiological (5%) Oxygen Concentrations. Front. Oncol. 2018, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 1 July 2021).
- Hamilton, N. Ggtern: An Extension to ‘ggplot2’ for the Creation of Ternary Diagrams. R Package Version 2.2.2. 2018. Available online: https://cran.r-project.org/web/packages/ggtern/index.html (accessed on 1 July 2021).
- New Zealand Winegrowers. Annual Report 2020; New Zealand Winegrowers: Auckland, New Zealand, 2020; Available online: https://www.nzwine.com/en/media/statistics/annual-report (accessed on 6 June 2022).
- New Zealand Winegrowers. Annual Report 2021; New Zealand Winegrowers: New Zealand, Auckland, 2021; Available online: https://www.nzwine.com/en/media/statistics/annual-report (accessed on 6 June 2022).
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Jiang, W.B.; Xie, M.X. Flavonoids: Recent Advances as Anticancer Drugs. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 152–164. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Medina-Juárez, L.A.; Soto-Valdez, H.; Manzanares-López, F.; Gámez-Meza, N. Influence of the Solvent System on the Composition of Phenolic Substances and Antioxidant Capacity of Extracts of Grape (Vitis vinifera L.) Marc. Aust. J. Grape Wine Res. 2014, 20, 208–213. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction Yields and Anti-Oxidant Activity of Proanthocyanidins from Different Parts of Grape Pomace: Effect of Mechanical Treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Amico, V.; Chillemi, R.; Mangiafico, S.; Spatafora, C.; Tringali, C. Polyphenol-Enriched Fractions from Sicilian Grape Pomace: HPLC–DAD Analysis and Antioxidant Activity. Bioresour. Technol. 2008, 99, 5960–5966. [Google Scholar] [CrossRef]
- Chafer, A.; Pascual-Martí, M.C.; Salvador, A.; Berna, A. Supercritical Fluid Extraction and HPLC Determination of Relevant Polyphenolic Compounds in Grape Skin. J. Sep. Sci. 2005, 28, 2050–2056. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of Phenolics from Vitis Vinifera Wastes Using Non-Conventional Techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carullo, G.; Ramunno, A.; Sommella, E.M.; De Luca, M.; Belsito, E.L.; Frattaruolo, L.; Brindisi, M.; Campiglia, P.; Cappello, A.R.; Aiello, F. Ultrasound-Assisted Extraction, Chemical Characterization, and Impact on Cell Viability of Food Wastes Derived from Southern Italy Autochthonous Citrus Fruits. Antioxidants 2022, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.A.; Amendola, D.; Maggi, L.; Zalacain, A.; De, F.D.M.; Spigno, G. Microwave-Assisted Extraction of Phenolic Compounds from Dried Waste Grape Skins. Int. J. Food Eng. 2015, 11, 359–370. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, D.; Xu, M.; Chen, J. Microwave-Assisted Extraction of Polyphenols from Camellia Oleifera Fruit Hull. Molecules 2011, 16, 4428–4437. [Google Scholar] [CrossRef] [Green Version]
- Da Porto, C.; Natolino, A. Supercritical Fluid Extraction of Polyphenols from Grape Seed (Vitis Vinifera): Study on Process Variables and Kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Vergara, D.; Domenico, S.D.; Tinelli, A.; Stanca, E.; Mercato, L.L.D.; Giudetti, A.M.; Simeone, P.; Guazzelli, N.; Lessi, M.; Manzini, C.; et al. Anticancer Effects of Novel Resveratrol Analogues on Human Ovarian Cancer Cells. Mol. BioSyst. 2017, 13, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Yang, C.; Zhang, L.; Wang, S.; Ma, M.; Zhao, J.; Song, Z.; Wang, F.; Qu, X.; Li, F.; et al. Development of M10, Myricetin-3-O-β-d-Lactose Sodium Salt, a Derivative of Myricetin as a Potent Agent of Anti-Chronic Colonic Inflammation. Eur. J. Med. Chem. 2019, 174, 9–15. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.-J.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Peracetylation as a Means of Enhancing in Vitro Bioactivity and Bioavailability of Epigallocatechin-3-Gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef] [Green Version]
- Walle, T.; Wen, X.; Walle, U.K. Improving Metabolic Stability of Cancer Chemoprotective Polyphenols. Expert Opin. Drug Metab. Toxicol. 2007, 3, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Tang, X.; Li, H.; Chen, H.; Yu, S. Molecular Hybridization of Grape Seed Extract: Synthesis, Structural Characterization and Anti-Proliferative Activity in Vitro. Food Res. Int. 2020, 131, 109005. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Empirical Parameters of Solvent Polarity. In Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 425–508. ISBN 978-3-527-32473-6. [Google Scholar]
- World Cancer Research Fund International. Cancer Data|World Cancer Research Fund International. WCRF International. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data (accessed on 10 June 2022).
- Omonga, N.; Zia, Z.; Ghanbour, H.; Ragazzon-Smith, A.; Foster, H.; Hadfield, J.; Ragazzon, P. Facile Synthesis and Biological Evaluation of Chrysin Derivatives. J. Chem. Res. 2021, 45, 1083–1092. [Google Scholar] [CrossRef]
- Nair, S.V.G.; Ziaullah; Rupasinghe, H.P.V. Fatty Acid Esters of Phloridzin Induce Apoptosis of Human Liver Cancer Cells through Altered Gene Expression. PLoS ONE 2014, 9, e107149. [Google Scholar] [CrossRef] [PubMed]






| Acetone:H2O:EtOH | ||||||
|---|---|---|---|---|---|---|
| 80:20:0 | 60:30:10 | 50:30:20 | 40:40:20 | 30:60:10 | 30:20:50 | 20:50:30 |
| 70:30:0 | 60:20:20 | 50:20:30 | 40:30:30 | 30:50:20 | 20:80:0 | 20:40:40 |
| 60:40:0 | 50:50:0 | 40:60:0 | 40:20:40 | 30:40:30 | 20:70:10 | 20:30:50 |
| 60:30:10 | 50:40:10 | 40:50:10 | 30:70:0 | 30:30:40 | 20:60:20 | 20:20:60 |
| Grape Type (Component) | Extraction Technique (Conditions) | Solvent System | Flavonoid | Average Amount (mg/kg Pomace) | Reference |
|---|---|---|---|---|---|
| Sauvignon blanc (whole pomace) | SLE (r.t., 1 h) | Acetone:H2O:EtOH (50:50:0) | 1 | 466.29 (±83.01) | [30] |
| Acetone:H2O:EtOH (30:50:20) | 2 | 151.14 (±7.91) | |||
| Acetone:H2O:EtOH (40:30:30) | 3 | 31.89 (±2.77) * | |||
| Weisser Riesling (skins) | SLE (r.t., 2 h) | MeOH:HCl (99.9:0.1 v/v) | 1 | 226.7 (±24.6) dw | [45] |
| 2 | 134.6 (±12.1) dw | ||||
| Weisser Riesling (seeds) | 1 | 790.2 (±11.2) dw | |||
| 2 | 674.5 (±24.9) dw | ||||
| Pinot noir (seed) | SLE (25 °C, 19 h) | MeOH | 1 | 1583 dw | [46] |
| 3 | 1386 dw | ||||
| EtOH | 1 | 1450 dw | |||
| 3 | 1386 dw |
| Flavonoid | Best Solvent System (Acetone:H2O:EtOH) | Average Quantity Extracted (mg/kg Pomace) |
|---|---|---|
| 1 | 80:20:0 | 174.1 (±17.1) |
| 2 | 40:40:20 | 269.6 (±34.1) |
| 3 | 40:50:10 | 87.0 (±46.4) * |
| Acetone:H2O:EtOH | (+)-Catechin (1) | (−)-Epicatechin (2) | Quercetin (3) | |||
|---|---|---|---|---|---|---|
| mg/kg Pomace | Rank | mg/kg Pomace | Rank | mg/kg Pomace | Rank | |
| 60:30:10 | 148.4 (±25.7) | 16 | 218.8 (±39.5) | 16 | 49.0 (±14.3) | 16 |
| 50:40:10 | 157.0 (±32.1) | 10 | 231.1 (±39) | 12 | 66.5 (±4.2) | 4 |
| 50:30:20 | 165.9 (±16.3) | 7 | 250.86 (±25.3) | 5 | 72.3 (±39.1) | 2 |
| 40:50:10 | 169.9 (±28.3) | 3 | 227.1 (±18.8) | 14 | 87.0 (±46.4) | 1 |
| 40:40:20 | 173.3 (±23.9) | 2 | 269.59 (±34.1) | 1 | 55.0 (±1.3) | 9 |
| 40:30:30 | 164.7 (±18.4) | 8 | 240.1 (±8.9) | 9 | 58.9 (±01.0) | 7 |
| Flavonoid | Extraction on Small-Scale Pomace (mg/kg Pomace) | Extraction on Large-Scale Pomace (mg/kg Pomace) |
|---|---|---|
| 1 | 164.7 (±18.4) | 14.0 (±5.3) |
| 2 | 240.1 (±8.9) | 1.1 (±0.6) |
| 3 | 54.9 (±1.0) * | 29.7 (±1.1) * |
| Crude Extract Type | % Cell Proliferation (Compared to Control) | |
|---|---|---|
| HCT116 | MDA-MB-231 | |
| Non-derivatized | 95.6 (±2.2) | 75.1 (±27.8) |
| Octanoyl | 91.9 (±4.1) | 82.6 (±21.0) |
| Lauroyl | 91.4 (±4.2) | 88.9 (±15.0) |
| Palmitoyl | 88.6 (±6.6) | 75.8 (±24.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.; Pilkington, L.I.; Barker, D.; Fedrizzi, B. Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods. Foods 2022, 11, 1999. https://doi.org/10.3390/foods11141999
Lo S, Pilkington LI, Barker D, Fedrizzi B. Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods. Foods. 2022; 11(14):1999. https://doi.org/10.3390/foods11141999
Chicago/Turabian StyleLo, Stephen, Lisa I. Pilkington, David Barker, and Bruno Fedrizzi. 2022. "Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods" Foods 11, no. 14: 1999. https://doi.org/10.3390/foods11141999
APA StyleLo, S., Pilkington, L. I., Barker, D., & Fedrizzi, B. (2022). Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods. Foods, 11(14), 1999. https://doi.org/10.3390/foods11141999