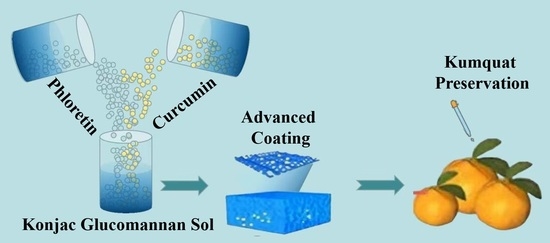
Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Konjac Glucomannan Coatings
2.3. Characterization of Konjac Glucomannan Coatings
2.4. Antioxidant Activity of Konjac Glucomannan Coatings
2.5. Antibacterial Activity of Konjac Glucomannan Coatings
2.6. The Preservation Experiment on Kumquats
2.7. Statistical Analysis
3. Results
3.1. Characterization of Konjac Glucomannan Coatings
3.2. Antioxidant Activity Assay of Coatings
3.3. Antibacterial Activity Assay of Coatings
3.4. The Preservation Effect of Kumquats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santhosh, R.; Nath, D.; Sarkar, P. Novel food packaging materials including plant-based byproducts: A review. Trends Food Sci. Technol. 2021, 118, 471–489. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Antimicrobial and Antioxidant Properties of Polyvinyl Alcohol Bio Composite Films Containing Seaweed Extracted Cellulose Nanocrystal and Basil Leaves Extract. Int. J. Biol. Macromol. 2018, 107, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple Peel Extract Incorporated Poly (Vinyl Alcohol)-Corn Starch Film for Active Food Packaging: Preparation, Characterization and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J.; et al. Advanced Konjac Glucomannan-Based Films in Food Packaging: Classification, Preparation, Formation Mechanism and Function. LWT 2021, 152, 112338. [Google Scholar] [CrossRef]
- Yu, S.H.; Hsieh, H.Y.; Pang, J.C.; Tang, D.W.; Shih, C.M.; Tsai, M.L.; Tsai, Y.C.; Mi, F.L. Active Films from Water-Soluble Chitosan/Cellulose Composites Incorporating Releasable Caffeic Acid for Inhibition of Lipid Oxidation in Fish Oil Emulsions. Food Hydrocoll. 2013, 32, 9–19. [Google Scholar] [CrossRef]
- He, S.; Gu, C.; Wang, D.; Xu, W.; Wang, R.; Ma, Y. The stability and In Vitro Digestion of Curcumin Emulsions Containing Konjac Glucomannan. LWT 2019, 117, 108672. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of Curcumin Using Coconut Milk Whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, B.-K.; Lee, M.H. Improving Curcumin Retention in Oil-In-Water Emulsions Coated by Chitosan and Their Disperse Stability Exposed to Thermal Treatments. J. Food Eng. 2021, 319, 110918. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, Characterization and Antioxidant Properties of Cur-Cumin Encapsulated Chitosan/Lignosulfonate Micelles. Carbohyd. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and Antimicrobial Activity of Phloretin and Its Glycosilated Derivatives Present in Apple and Kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- He, J.R.; Zhu, J.J.; Yin, S.W.; Yang, X.Q. Bioaccessibility and Intracellular Antioxidant Activity of Phloretin Embodied by Gliadin/Sodium Carboxymethyl Cellulose Nanoparticles. Food Hydrocoll. 2022, 122, 107076. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, L.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Physical, Structural, and Water Barrier Properties of Emulsified Blend Film Based on Konjac Glucomannan/Agar/Gum Arabic Incorporating Virgin Coconut Oil. LWT 2021, 154, 112683. [Google Scholar] [CrossRef]
- Ni, Y.; Shi, S.; Li, M.; Zhang, L.; Yang, C.; Du, T.; Wang, S.; Nie, H.; Sun, J.; Zhang, W.; et al. Visible Light Responsive, Self-Activated Bionanocomposite Films with Sustained Antimicrobial Activity for Food Packaging. Food Chem. 2021, 362, 130201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. Antioxidant Activities of Chlorogenic Acid Derivatives with Different Acyl Donor Chain Lengths and Their Stabilities During In Vitro Simulated Gastrointestinal Digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef]
- Gao, N.; Cui, H.; Lang, Y.; Zhang, W.; Shu, C.; Wang, Y.; Bian, Y.; Li, D.; Li, B. Conversion of Condensed Tannin from Chokeberry to Cyanidin: Evaluation of Antioxidant Activity and Gut Microbiota Regulation. Food Res. Int. 2022, 158, 111456. [Google Scholar] [CrossRef]
- Ni, Y.; Nie, H.; Wang, J.; Lin, J.; Wang, Q.; Sun, J.; Zhang, W.; Wang, J. Enhanced Functional Properties of Chitosan Films Incorporated with Curcumin-Loaded Hollow Graphitic Carbon Nitride Nanoparticles for Bananas Preservation. Food Chem. 2022, 366, 130539. [Google Scholar] [CrossRef]
- Guo, J.; Qin, D.; Li, W.; Wu, F.; Li, L.; Liu, X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021, 343, 109090. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Liu, Y.; Liu, H.; Shang, Y. Evaluating Effects of Ellagic Acid on the Quality of Kumquat Fruits During Storage. Sci. Hortic. 2018, 227, 244–254. [Google Scholar] [CrossRef]
- Tong, C.; Wu, Z.; Sun, J.; Lin, L.; Wang, L.; Guo, Y.; Huang, Z.; Wu, C.; Pang, J. Effect of Carboxylation Cellulose Nanocrystal and Grape Peel Extracts on the Physical, Mechanical and Antioxidant Properties of Konjac Glucomannan Films. Int. J. Biol. Macromol. 2020, 156, 874–884. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Antioxidant, Antibacterial and Anti-Cancer Activities of Β-And Γ-Cds/Curcumin Loaded in Chitosan Nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, R.; Li, X.L.; Liu, W.J.; Cheng, J.S.; Wang, W. Preparation of Pickering Emulsion Gels Based on Κ-Carrageenan and Covalent Crosslinking with EDC: Gelation Mechanism and Bioaccessibility of Curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and Its Uses in Active and Smart Food Packaging Applications-A Comprehensive Review. Food Chem. 2021, 375, 131885. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Qi, D.; Xia, L.; Li, L.; Li, X.; Jiang, S. Multifunctional Colorimetric Cellulose Acetate Membrane Incorporated with Perilla frutescens (L.) Britt. Anthocyanins And Chamomile Essential Oil. Carbohydr. Polym. 2022, 278, 118914. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, Antimutagenic and Antibacterial Activities of Curcumin-Β-Diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Jiang, H.; Li, Y.; Pang, J. Construction of Carboxymethyl Konjac Glucomannan/Chitosan Complex Nanogels as Potential Delivery Vehicles for Curcumin. Food Chem. 2021, 362, 130242. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus Bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Kumarihami, H.P.C.; Kim, Y.H.; Kwack, Y.B.; Kim, J.; Kim, J.G. Application of Chitosan as Edible Coating to Enhance Storability and Fruit Quality of Kiwifruit: A Review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, X.; Qi, H. Characterization and Bioactivity of Phlorotannin Loaded Protein-Polysaccharide Nanocomplexes. LWT 2021, 155, 112998. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, M.; Hu, S.; Sun, J.; Zhu, M.; Ni, Y.; Wang, J. Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation. Foods 2022, 11, 2363. https://doi.org/10.3390/foods11152363
Li M, Wang M, Hu S, Sun J, Zhu M, Ni Y, Wang J. Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation. Foods. 2022; 11(15):2363. https://doi.org/10.3390/foods11152363
Chicago/Turabian StyleLi, Mile, Mengyi Wang, Shuhui Hu, Jing Sun, Mingqiang Zhu, Yongsheng Ni, and Jianlong Wang. 2022. "Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation" Foods 11, no. 15: 2363. https://doi.org/10.3390/foods11152363
APA StyleLi, M., Wang, M., Hu, S., Sun, J., Zhu, M., Ni, Y., & Wang, J. (2022). Advanced Coatings with Antioxidant and Antibacterial Activity for Kumquat Preservation. Foods, 11(15), 2363. https://doi.org/10.3390/foods11152363