Characterization, Stability, and Antibrowning Effects of Oxyresveratrol Cyclodextrin Complexes Combined Use of Hydroxypropyl Methylcellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Solubilization Effect of Water-Soluble Polysaccharides on Oxy
2.3. Phase Solubility Study
2.4. Preparation of Oxy–HPMC, Oxy–CD, and Oxy–CD–HPMC Solutions
2.5. Fluorescence Spectroscopy Analysis
2.6. Particle Size Analysis
2.7. Stability Study of Oxy–CD and Oxy–CD–HPMC Solutions
2.8. Antibrowning Effects of Oxy–CD–HPMC and Oxy–CD–HPMC + VC on Fresh-Cut Apple Slices
2.9. Statistical Analysis
3. Results and Discussion
3.1. Solubility of Oxy in Various Water-Soluble Polymers
3.2. Study on the Interaction among Oxy–CD, and HPMC
3.2.1. Phase Solubility Study
3.2.2. Fluorescence Spectroscopy Analysis
3.2.3. Particle Size Analysis
3.3. Long-Term Storage
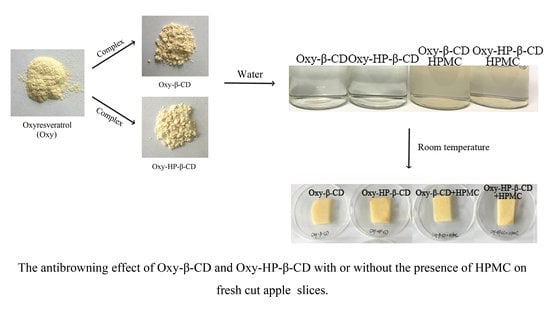
3.3.1. Visual Appearance
3.3.2. The Stability of Oxy in Water
3.3.3. The Stability of Oxy in Acidic Solution
3.4. Anti-Browning Effects of Oxy–CD–HPMC and Oxy–CD–HPMC–VC on Fresh-Cut Apple Slices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Likhitwitayawuid, K. Oxyresveratrol: Sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Sangsen, Y.; Sooksawate, T.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanapatapee, R. A self-microemulsifying formulation of oxyresveratrol prevents amyloid beta protein-induced neurodegeneration in mice. Planta Med. 2018, 84, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, Y.; Wang, X.; Jiang, X.; Zou, J.; Huang, R. Effects of the natural compound, oxyresveratrol, on the growth of Streptococcus mutans, and on biofilm formation, acid production, and virulence gene expression. Eur. J. Oral Sci. 2020, 128, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, Y.; Ji, Z.; Li, L.; Zhou, L. Pharmacological biotargets and the molecular mechanisms of oxyresveratrol treating colorectal cancer: Network and experimental analyses. BioFactors 2020, 46, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Guerrero-Rubio, M.A.; Caldera, F.; Cecone, C.; Trotta, F.; García-Carmona, F.; López-Nicolás, J.M. Lifespan extension in Caenorhabditis elegans by oxyresveratrol supplementation in hyper-branched cyclodextrin-based nanosponges. Int. J. Pharm. 2020, 589, 119862. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; Conesa, I.; Munoz-Sanchez, I.; Laveda-Cano, L.; Cano-Yelo, D.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of juice and milk “food models” fortified with oxyresveratrol and β-Cyclodextrin. Food Hydrocoll. 2020, 98, 105250. [Google Scholar] [CrossRef]
- Hu, N.; Mei, M.; Ruan, J.; Wu, W.; Wang, Y.; Yan, R. Regioselective glucuronidation of oxyresveratrol, a natural hydroxystilbene, by human liver and intestinal microsomes and recombinant ugts. Drug Metab. Pharmacokinet. 2014, 29, 229–236. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Molecular encapsulation and bioactivity of gnetol, a resveratrol analogue, for use in foods. J. Sci. Food Agric. 2022, 102, 4296–4303. [Google Scholar] [CrossRef]
- Jin, J.H.; Jiang, Y.Y.; Wang, Y.; Meng, Z.W.; Li, D.H.; Zhang, L.; Wang, H.; Zhang, Y.J. Oxyresveratrol-induced activation of Nrf2/HO-1 signaling pathway enhances ability of resveratrol to inhibit UVB-induced melanin. Int. J. Dermatol. Venereol. 2021, 4, 152–162. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Lipophilization of resveratrol and effects on antioxidant activities. J. Agric. Food Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Guo, Q.; Liu, Y.; Zhao, Y.; Wu, Y.; Sun, B.; Wang, J.; Liu, J.; Han, J. Investigation of binary and ternary systems of human serum albumin with oxyresveratrol/piceatannol and/or mitoxantrone by multipectroscopy, molecular docking and cytotoxicity evaluation. J. Mol. Liq. 2020, 311, 113364. [Google Scholar] [CrossRef]
- Li, J.; Lin, Z.; Tang, X.; Liu, G.; Chen, Y.; Zhai, X.; Huang, Q.; Cao, Y. Oxyresveratrol extracted from Artocarpus heterophyllus Lam. inhibits tyrosinase and age pigments in vitro and in vivo. Food Funct. 2020, 11, 6595–6607. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2022, 1–19, ahead-of-print. [Google Scholar] [CrossRef]
- Lv, X.; Cong, Z.; Liu, Z.; Ma, X.; Xu, M.; Tian, Y.; Zhang, X.; Xu, B.; Zhang, J.; Tang, Z. Improvement of the solubility, photostability, antioxidant activity and UVB photoprotection of trans-resveratrol by essential oil based microemulsions for topical application. J. Drug Deliv. Sci. Technol. 2018, 48, 346–354. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- He, J.; Zhu, Q.; Dong, X.; Pan, H.; Chen, J.; Zheng, Z.P. Oxyresveratrol and ascorbic acid O/W microemulsion: Preparation, characterization, anti-isomerization and potential application as antibrowning agent on fresh-cut lotus root slices. Food Chem. 2017, 214, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, K.Y.; Ho, P.C. Formulation and optimization of zinc-pectinate beads for the controlled delivery of resveratrol. AAPS PharmSciTech 2010, 11, 729–742. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact. Mater. 2021, 14, 182–205. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Iswandana, R.; Jufri, M. Preparation, characterization, and formulation of solid lipid nanoparticles lotion from mulberry roots (Morus alba L.). Int. J. Appl. Pharm 2020, 12, 182–186. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- He, J.; Guo, F.; Lin, L.; Chen, H.; Chen, J.; Cheng, Y.; Zheng, Z. Investigating the oxyresveratrol β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin complexes: The effects on oxyresveratrol solution, stability, and antibrowning ability on fresh grape juice. LWT 2019, 100, 263–270. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Complexation of resveratrol by native and modified cyclodextrins: Determination of complexation constant by enzymatic, solubility and fluorimetric assays. Food Chem. 2008, 111, 262–267. [Google Scholar] [CrossRef]
- Zhang, X.; Le, Y.; Wang, J.; Zhao, H.; Chen, J. Resveratrol nanodispersion with high stability and dissolution rate. LWT 2013, 50, 622–628. [Google Scholar] [CrossRef]
- Jug, M.; Bećirević-Laćan, M. Multicomponent complexes of piroxicam with cyclodextrins and hydroxypropyl methylcellulose. Drug Dev. Ind. Pharm. 2004, 30, 1051–1060. [Google Scholar] [CrossRef]
- Sultan, A.; Imam, S.S.; Hussain, A.; Altamimi, M.A. Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes 2020, 8, 1450. [Google Scholar] [CrossRef]
- Anwer, M.K.; Jamil, S.; Ansari, M.J.; Al-Shdefat, R.; Ali, B.E.; Ganaie, M.A.; Abdel-Kadercd, M.S.; Shakeel, F. Water soluble binary and ternary complexes of diosmin with β-cyclodextrin: Spectroscopic characterization, release studies and anti-oxidant activity. J. Mol. Liq. 2014, 199, 35–41. [Google Scholar] [CrossRef]
- Jadhav, A.T.; Burade, K.B.; Kulkarni, D.A.; Pekamwar, S.S.; Kulkarni, S.S. Development and evaluation of drug-cyclodextrin-polymer ternary system of Cefuroxime axetil to enhance the solubility and dissolution efficiency. Res. J. Pharm. Technol. 2022, 15, 779–786. [Google Scholar] [CrossRef]
- Homayouni, A.; Sohrabi, M.; Amini, M.; Varshosaz, J.; Nokhodchi, A. Effect of high pressure homogenization on physicochemical properties of curcumin nanoparticles prepared by antisolvent crystallization using HPMC or PVP. Mater. Sci. Eng. C 2019, 98, 185–196. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin multicomponent complexes: Pharmaceutical applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Wang, F.; Chatterjee, S. Dominant carbons in trans-and cis-resveratrol isomerization. J. Phys. Chem. B 2017, 121, 4745–4755. [Google Scholar] [CrossRef]
- Maafi, M.; Al-Qarni, M.A. Φ-order spectrophotokinetic characterisation and quantification of trans-cis oxyresveratrol reactivity, photodegradation and actinometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 64–71. [Google Scholar] [CrossRef]
- Francioso, A.; Laštovičková, L.; Mosca, L.; Boffi, A.; Bonamore, A.; Macone, A. Gas Chromatographic–Mass Spectrometric method for the simultaneous determination of resveratrol isomers and 2, 4, 6-trihydroxyphenanthrene in red wines exposed to UV-Light. J. Agric. Food Chem. 2019, 67, 11752–11757. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Waterhouse, A.L.; Torre-Boronat, M.C. Levels of cis- and trans-Resveratrol and Their Glucosides in White and Rosé Vitis vinifera Wines from Spain. J. Agric. Food Chem. 1996, 44, 2124–2128. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Mo, L.; Zou, Y.; Zhao, G. Tyrosinase inhibitory mechanism and the anti-browning properties of piceid and its ester. Food Chem. 2022, 390, 133207. [Google Scholar] [CrossRef]
- Gheysarbigi, S.; Mirdehghan, S.H.; Ghasemnezhad, M.; Nazoori, F. The inhibitory effect of nitric oxide on enzymatic browning reactions of in-package fresh pistachios (Pistacia vera L.). Postharvest Biol. Technol. 2020, 159, 110998. [Google Scholar] [CrossRef]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K.K. Effect of HPMC concentration on β-cyclodextrin solubilization of norfloxacin. Carbohydr. Polym. 2014, 101, 505–510. [Google Scholar] [CrossRef]
- Valero, M.; Pérez-Revuelta, B.I.; Rodríguez, L.J. Effect of PVP K-25 on the formation of the naproxen: β-ciclodextrin complex. Int. J. Pharm. 2003, 253, 97–110. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation mechanism of oxyresveratrol by β-cyclodextrin and hydroxypropyl-β-cyclodextrin and computational analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, R.; Ge, X.; Cheng, J. Complexation of capsaicin with hydroxypropyl-β-cyclodextrin and its analytical application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117278. [Google Scholar] [CrossRef]
- Lee, S.C.; Heo, J.; Woo, H.C.; Lee, J.A.; Seo, Y.H.; Lee, C.L.; Sehoon, K.; Kwon, O.P. Fluorescent molecular rotors for viscosity sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Rades, T. Influence of polymer addition on the amorphization, dissolution and physical stability of co-amorphous systems. Int. J. Pharm. 2020, 588, 119768. [Google Scholar] [CrossRef]
- Guo, Y.; Jauregi, P. Protective effect of β-lactoglobulin against heat induced loss of antioxidant activity of resveratrol. Food Chem. 2018, 266, 101–109. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Li, J.; Subirade, M.; Song, Y.; Liang, L. Effect of resveratrol or ascorbic acid on the stability of α-tocopherol in O/W emulsions stabilized by whey protein isolate: Simultaneous encapsulation of the vitamin and the protective antioxidant. Food Chem. 2016, 196, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Pronkin, P.; Tatikolov, A. Isomerization and properties of isomers of carbocyanine dyes. Sci 2019, 1, 19. [Google Scholar] [CrossRef]
- Fan, N.; Ma, P.; Wang, X.; Li, C.; Zhang, X.; Zhang, K.; Li, J.; He, Z. Storage stability and solubilization ability of HPMC in curcumin amorphous solid dispersions formulated by Eudragit E100. Carbohydr. Polym. 2018, 199, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funt. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 96, 196–204. [Google Scholar] [CrossRef]
- Li, H.; Cheng, K.; Cho, C.; He, Z.; Wang, M. Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples. J. Agric. Food Chem. 2007, 55, 2604–2610. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, S.M.; Jeong, I.; Heo, W.; Hwang, K.A.; Han, B.K.; Kim, Y.J. Anti-browning and oxidative enzyme activity of rice bran extract treatment on freshly cut ‘Fuji’ apple. Agronomy 2022, 12, 86. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S.; Desa, S.; Chen, S.N. Use of Centella asiatica extract in reducing microbial contamination and browning effect in fresh cut fruits and vegetables during storage: A potential alternative of synthetic preservatives. LWT 2021, 151, 112229. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Soares, N.F.F.; Silva, D.F.P.; Camilloto, G.P.; Oliveira, C.P.; Pinheiro, N.M.; Medeiros, E.A.A. Antimicrobial edible coating in post-harvest conservation of guava. Rev. Bras. Frutic. 2011, 33, 281–289. [Google Scholar] [CrossRef]











| Test Solution | Composition |
|---|---|
| Water | Water |
| β-CD | 0.4 mg/mL β-CD |
| HP–β-CD | 0.4 mg/mL HP–β-CD |
| HPMC | 0.1 mg/mL HPMC |
| VC | 0.5 mg/mL VC |
| Oxy–β-CD | 0.5 mg/mL Oxy–β-CD inclusion complex |
| Oxy–HP–β-CD | 0.5 mg/mL Oxy–HP–β-CD inclusion complex |
| Oxy–β-CD + VC | 0.5 mg/mL Oxy–β-CD inclusion complex and 0.5 mg/mL VC |
| Oxy–HP–β-CD + VC | 0.5 mg/mL Oxy–HP–β-CD inclusion complex and 0.5 mg/mL VC |
| VC + HPMC | 0.5 mg/mL VC and 0.1 mg/mL HPMC |
| Oxy–β-CD + HPMC | 0.5 mg/mL Oxy–β-CD inclusion complex and 0.1 mg/mL HPMC |
| Oxy–HP–β-CD + HPMC | 0.5 mg/mL Oxy–HP–β-CD inclusion complex and 0.1 mg/mL HPMC |
| Oxy–β-CD + VC + HPMC | 0.5 mg/mL Oxy–β-CD inclusion complex, 0.5 mg/mL VC and 0.1 mg/mL HPMC |
| Oxy–HP–β-CD + VC + HPMC | 0.5 mg/mL Oxy–HP–β-CD inclusion complex, 0.5 mg/mL VC and 0.1 mg/mL HPMC |
| Water | |||||||
|---|---|---|---|---|---|---|---|
| Time (Day) | Oxy | Oxy–β-CD | Oxy–HP–β-CD | Oxy–HPMC | Oxy–β-CD-HPMC | Oxy–HP–β-CD-HPMC | |
| Total Oxy | 0 | 100 ± 6.50 a | 100 ± 0.69 a | 100 ± 4.03 a | 100 ± 3.48 a | 100 ± 0.86 a | 100 ± 0.54 a |
| 1 | 96.19 ± 2.85 a | 92.60 ± 12.36 a | 99.04 ± 8.49 a | 94.39 ± 0.29 a | 93.02 ± 0.90 a | 94.93 ± 3.29 a | |
| 3 | 100.07 ± 4.05 a | 106.24 ± 2.69 a | 104.39 ± 0.82 a | 87.28 ± 0.74 b | 87.37 ± 1.28 b | 90.22 ± 2.10 b | |
| 7 | 101.81 ± 4.19 a | 98.01 ± 3.02 a | 101.21 ± 0.81 a | 82.33 ± 9.89 b | 92.53 ± 10.14 ab | 91.71 ± 10.11 ab | |
| 14 | 95.20 ± 1.31 a | 93.97 ± 1.36 a | 99.50 ± 1.38 a | 79.51 ± 4.90 b | 91.25 ± 8.00 a | 93.15 ± 6.47 a | |
| 30 | 72.91 ± 1.04 a | 84.71 ± 0.28 a | 93.93 ± 0.65 b | 60.01 ± 2.00 a b | 80.22 ± 0.87 a | 84.12 ± 4.72 ab | |
| Trans-Oxy | 0 | 100 ± 6.50 a | 100 ± 0.69 a | 100 ± 4.03 a | 100 ± 3.48 a | 100 ± 0.86 a | 100 ± 0.54 a |
| 1 | 54.27 ± 1.57 c | 59.87 ± 6.06 c | 76.28 ± 4.37 b | 94.39 ± 0.29 a | 93.02 ± 0.90 a | 94.93 ± 3.29 a | |
| 3 | 15.22 ± 2.09 b | 19.81 ± 1.86 b | 23.44 ± 4.26 b | 87.28 ± 0.74 a | 87.37 ± 1.28 a | 89.14 ± 1.99 a | |
| 7 | 32.02 ± 2.66 b | 24.23 ± 0.70 b | 30.55 ± 0.40 b | 82.33 ± 9.89 a | 69.01 ± 8.24 a | 69.98 ± 8.89 a | |
| 14 | 17.35 ± 0.32 c | 20.71 ± 0.59 c | 30.21 ± 0.65 b | 79.51 ± 4.90 a | 77.05 ± 6.73 a | 76.04 ± 5.21 a | |
| 30 | 9.04 ± 0.17d | 13.23 ± 0.18 cd | 21.30 ± 0.48 c | 60.01 ± 2.00 b | 70.01 ± 0.24 a | 74.01 ± 4.65 a | |
| Cis-Oxy | 0 | ND | ND | ND | ND | ND | ND |
| 1 | 41.92 ± 1.28 a | 32.73 ± 6.30 b | 22.76 ± 4.13 c | ND | ND | ND | |
| 3 | 84.85 ± 1.96 a | 82.44 ± 0.83 a | 80.95 ± 8.05 a | ND | ND | 1.08 ± 0.11 b | |
| 7 | 69.79 ± 1.52 a | 73.78 ± 2.32 a | 70.66 ± 0.41 a | ND | 23.52 ± 1.9 b | 21.73 ± 1.23 b | |
| 14 | 77.85 ± 1.00 a | 73.26 ± 0.76 a | 69.28 ± 0.72 b | ND | 14.21 ± 1.28 c | 17.11 ± 1.26 c | |
| 30 | 63.87 ± 0.87 b | 71.48 ± 0.10 a | 72.64 ± 0.17 a | ND | 10.21 ± 0.63 c | 10.11 ± 0.07 c | |
| Acidic solution (pH 3.6) | |||||||
| Time (day) | Oxy | Oxy–β-CD | Oxy–HP–β-CD | Oxy–HPMC | Oxy–β-CD-HPMC | Oxy–HP–β-CD-HPMC | |
| Total Oxy | 0 | 100 ± 0.95 a | 100 ± 0.02 a | 100 ± 0.06 a | 100 ± 2.37 a | 100 ± 0.21 a | 100 ± 0.88 a |
| 1 | 70.08 ± 3.69 c | 93.83 ± 2.67 ab | 90.16 ± 3.03 b | 100.30 ± 2.29 a | 100.72 ± 0.66 a | 101.04 ± 2.13 a | |
| 3 | 61.77 ± 0.80 b | 90.34 ± 1.29 a | 87.31 ± 1.06 a | 89.28 ± 0.84 a | 91.05 ± 1.24 a | 88.67 ± 1.28 a | |
| 7 | 56.42 ± 9.07 b | 89.52 ± 2.26 a | 86.61 ± 1.04 a | 88.69 ± 6.28 a | 85.24 ± 6.79 a | 90.94 ± 7.66 a | |
| 14 | 39.25 ± 0.55 b | 81.88 ± 4.60 a | 80.87 ± 1.19 a | 77.61 ± 5.73 a | 85.12 ± 4.81 a | 84.39 ± 5.68 a | |
| 30 | 31.94 ± 9.95 d | 74.59 ± 2.08 b | 77.33 ± 1.87 ab | 60.01 ± 7.03 c | 83.22 ± 4.43 a | 85.12 ± 5.98 a | |
| Trans-Oxy | 0 | 100 ± 0.95 a | 100 ± 0.02 a | 100 ± 0.06 a | 100 ± 2.37 a | 100 ± 0.21 a | 100 ± 0.88 a |
| 1 | 53.57 ± 2.00 c | 84.54 ± 1.29 b | 88.37 ± 1.47 b | 100.3 ± 2.29 a | 100.72 ± 0.66 a | 101.04 ± 2.13 a | |
| 3 | 34.56 ± 0.54 c | 77.1 ± 0.95 b | 83.43 ± 0.81 ab | 86.27 ± 0.79 a | 91.05 ± 1.24 a | 88.67 ± 1.28 a | |
| 7 | 45.33 ± 4.59 b | 90.11 ± 1.12 a | 91.12 ± 0.61 a | 84.64 ± 6.04 a | 85.24 ± 6.79 a | 90.94 ± 7.66 a | |
| 14 | 26.43 ± 0.44 c | 75.37 ± 2.25 b | 82.16 ± 0.76 a | 74.3 ± 5.40 b | 74.43 ± 12.52 b | 84.39 ± 5.68 a | |
| 30 | 31.94 ± 0.85 c | 74.59 ± 2.08 a | 77.33 ± 1.87 a | 60.01 ± 7.03 b | 80.01 ± 2.73 a | 80.01 ± 4.5 a | |
| Cis-Oxy | 0 | ND | ND | ND | ND | ND | ND |
| 1 | 16.51 ± 1.7 a | 9.28 ± 1.37 b | 1.79 ± 1.56 c | ND | ND | ND | |
| 3 | 27.21 ± 0.26 a | 13.25 ± 0.34 b | 3.88 ± 0.25 c | 3.01 ± 0.04 c | ND | ND | |
| 7 | 11.09 ± 4.48 a | 0 ± 1.14 c | 0 ± 0.43 c | 4.05 ± 0.24 b | ND | ND | |
| 14 | 12.83 ± 0.11 a | 6.51 ± 2.35 b | 0 ± 0.43 d | 3.31 ± 0.33 c | 10.7 ± 0.35 a | ND | |
| 30 | ND | ND | 0 ± 0.17 b | ND | 3.21 ± 1.70 a | 5.11 ± 1.48 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Chen, H.-Y.; Chen, H.; Wang, B.; Guo, F.; Zheng, Z.-P. Characterization, Stability, and Antibrowning Effects of Oxyresveratrol Cyclodextrin Complexes Combined Use of Hydroxypropyl Methylcellulose. Foods 2022, 11, 2471. https://doi.org/10.3390/foods11162471
He J, Chen H-Y, Chen H, Wang B, Guo F, Zheng Z-P. Characterization, Stability, and Antibrowning Effects of Oxyresveratrol Cyclodextrin Complexes Combined Use of Hydroxypropyl Methylcellulose. Foods. 2022; 11(16):2471. https://doi.org/10.3390/foods11162471
Chicago/Turabian StyleHe, Jianfei, Huai-Yu Chen, Hongbin Chen, Baobei Wang, Fengxian Guo, and Zong-Ping Zheng. 2022. "Characterization, Stability, and Antibrowning Effects of Oxyresveratrol Cyclodextrin Complexes Combined Use of Hydroxypropyl Methylcellulose" Foods 11, no. 16: 2471. https://doi.org/10.3390/foods11162471
APA StyleHe, J., Chen, H.-Y., Chen, H., Wang, B., Guo, F., & Zheng, Z.-P. (2022). Characterization, Stability, and Antibrowning Effects of Oxyresveratrol Cyclodextrin Complexes Combined Use of Hydroxypropyl Methylcellulose. Foods, 11(16), 2471. https://doi.org/10.3390/foods11162471