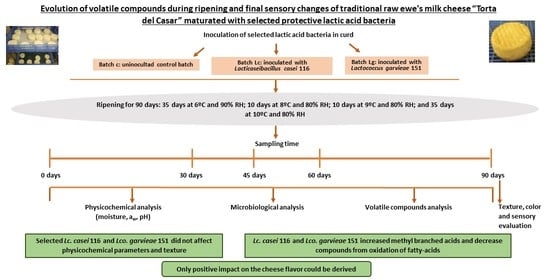
Evolution of Volatile Compounds during Ripening and Final Sensory Changes of Traditional Raw Ewe’s Milk Cheese “Torta del Casar” Maturated with Selected Protective Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Strains and Growth Conditions
2.2. Preparation of “Torta del Casar” Cheese
2.3. Microbiological Analysis
2.4. Physicochemical Analysis
2.5. Instrumental Texture
2.6. Instrumental Color
2.7. Volatile Compound Analysis
2.8. Sensory Evaluation
2.9. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. Enumeration of Microorganisms
3.3. Analysis of Volatile Compounds
3.3.1. Acids
3.3.2. Alcohols, Ketones and Aldehydes
3.3.3. Esters and Other Compounds
3.4. Texture and Colour Analysis
3.5. Sensory Evaluation
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commision Regulation (EC). Commision Regulation (EC) No 1491/2003 of 25 August 2003. Off. J. Eur. Union 2003, 10, 2002–2003. [Google Scholar]
- Crespo, A.; Jiménez, A.; Ruiz-Moyano, S.; Merchán, A.V.; Galván, A.I.; Benito, M.J.; Martín, A. Low-frequency ultrasound as a tool for quality control of soft-bodied raw ewe’s milk cheeses. Food Control 2022, 131, 108405. [Google Scholar] [CrossRef]
- Pereira, C.I.; Graça, J.A.; Ogando, N.S.; Gomes, A.M.P.; Xavier Malcata, F. Influence of bacterial dynamics upon the final characteristics of model Portuguese traditional cheeses. Food Microbiol. 2010, 27, 339–346. [Google Scholar] [CrossRef]
- Carrascosa, C.; Millán, R.; Saavedra, P.; Jaber, J.R.; Raposo, A.; Sanjuán, E. Identification of the risk factors associated with cheese production to implement the hazard analysis and critical control points (HACCP) system on cheese farms. J. Dairy Sci. 2016, 99, 2606–2616. [Google Scholar] [CrossRef]
- Martinez-Rios, V.; Dalgaard, P. Prevalence of Listeria monocytogenes in European cheeses: A systematic review and meta-analysis. Food Control 2018, 84, 205–214. [Google Scholar] [CrossRef]
- Amato, E.; Filipello, V.; Gori, M.; Lomonaco, S.; Losio, M.N.; Parisi, A.; Huedo, P.; Knabel, S.J.; Pontello, M. Identification of a major Listeria monocytogenes outbreak clone linked to soft cheese in Northern Italy—2009–2011. BMC Infect. Dis. 2017, 17, 342. [Google Scholar] [CrossRef]
- Possas, A.; Bonilla-Luque, O.M.; Valero, A. From cheese-making to consumption: Exploring the microbial safety of cheeses through predictive microbiology models. Foods 2021, 10, 355. [Google Scholar] [CrossRef]
- Rolim, F.R.L.; Freitas Neto, O.C.; Oliveira, M.E.G.; Oliveira, C.J.B.; Queiroga, R.C.R.E. Cheeses as food matrixes for probiotics: In vitro and in vivo tests. Trends Food Sci. Technol. 2020, 100, 138–154. [Google Scholar] [CrossRef]
- Gezginc, Y.; Karabekmez-Erdem, T.; Tatar, H.D.; Dağgeçen, E.C.; Ayman, S.; Akyol, İ. Metagenomics and volatile profile of Turkish artisanal Tulum cheese microbiota. Food Biosci. 2022, 45, 101497. [Google Scholar] [CrossRef]
- Martín, I.; Cordoba, J.J.; Alía, A.; Martínez, R.; Rodríguez, A. Selection and characterization of lactic acid bacteria from traditional ripened foods with activity against Listeria monocytogenes. LWT Food Sci. Technol. 2022, 163, 113579. [Google Scholar] [CrossRef]
- Bezerril, F.F.; Pimentel, T.C.; Marília da Silva Sant’Ana, A.; de Fátima Vanderlei de Souza, M.; Lucena de Medeiros, L.; Galvão, M.; Madruga, M.S.; de Cássia Ramos do Egypto Queiroga, R.; Magnani, M. Lacticaseibacillus casei 01 improves the sensory characteristics in goat milk yogurt added with xique-xique (Pilosocereus gounellei) jam through changes in volatiles concentration. LWT 2022, 154, 112598. [Google Scholar] [CrossRef]
- Fernández, E.; Alegría, Á.; Delgado, S.; Mayo, B. Phenotypic, genetic and technological characterization of Lactococcus garvieae strains isolated from a raw milk cheese. Int. Dairy J. 2010, 20, 142–148. [Google Scholar] [CrossRef]
- Abdelfatah, E.N.; Mahboub, H.H.H. Studies on the effect of Lactococcus garvieae of dairy origin on both cheese and Nile tilapia (O. niloticus). Int. J. Vet. Sci. Med. 2018, 6, 201–207. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Alía, A.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Córdoba, J.J. Control of Listeria monocytogenes growth and virulence in a traditional soft cheese model system based on lactic acid bacteria and a whey protein hydrolysate with antimicrobial activity. Int. J. Food Microbiol. 2022, 361, 109444. [Google Scholar] [CrossRef]
- Beaufort, A.; Cornu, M.; Bergis, H.; Lardeux, A.L.; Lombard, B. EURL Lm Technical Guiadance Document for Conducing Shelf-Life Studies on Listeria monocytogenes in Ready to Eat Foods. Available online: https://www.fsai.ie/uploadedFiles/EURL%20Lm_Technical%20Guidance%20Document%20Lm%20shelf-life%20studies_V3_2014-06-06%20(2).pdf (accessed on 26 July 2022).
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “salchichón” processing with a selected Lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef]
- Walter, J.; Tannock, G.W.; Tilsala-Timisjarvi, A.; Rodtong, S.; Loach, D.M.; Munro, K.; Alatossava, T. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 2000, 66, 297–303. [Google Scholar] [CrossRef]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Martín, I.; Pérez-Baltar, A.; Medina, M.; Córdoba, J.J. Prevalence and characterization of Listeria monocytogenes in deboning and slicing areas of Spanish dry-cured ham processing. LWT Food Sci. Technol. 2020, 128, 109498. [Google Scholar] [CrossRef]
- AOAC. Moisture in Malt Gravimetric Method (935.29), 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Portalo-Calero, F.; Arroyo, P.; Suárez, J.I.; Lozano, J. Triangular test of amanita mushrooms by using electronic nose and sensory panel. Foods 2019, 8, 414. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 2, 347–370. [Google Scholar] [CrossRef]
- Ordiales, E.; Martín, A.; Benito, M.J.; Hernández, A.; Ruiz-Moyano, S.; de Guía Córdoba, M. Role of the microbial population on the flavor of the soft-bodied cheese Torta del Casar. J. Dairy Sci. 2013, 96, 5477–5486. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, F.; Song, Y.; Lou, Y.; Zhao, A.; Liu, Y.; Peng, H.; Hui, Y.; Ren, R.; Wang, B. Physicochemical and textural characteristics and volatile compounds of semihard goat cheese as affected by starter cultures. J. Dairy Sci. 2021, 104, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Özer, E.; Kesenkaş, H. The effect of using different starter culture combinations on ripening parameters, microbiological and sensory properties of Mihaliç cheese. J. Food Sci. Technol. 2019, 56, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Ordiales, E.; Martín, A.; Benito, M.J.; Fernández, M.; Casquete, R.; de Guía Córdoba, M. Influence of the technological properties of vegetable rennet (Cynara cardunculus) on the physicochemical, sensory and rheological characteristics of “Torta del Casar” cheese. Int. J. Dairy Technol. 2014, 67, 402–409. [Google Scholar] [CrossRef]
- Ordiales, E.; Benito, M.J.; Martín, A.; Casquete, R.; Serradilla, M.J.; de Guía Córdoba, M. Bacterial communities of the traditional raw ewe’s milk cheese “Torta del Casar” made without the addition of a starter. Food Control 2013, 33, 448–454. [Google Scholar] [CrossRef]
- Gonçalves, M.T.P.; Benito, M.J.; de Córdoba, M.G.; Egas, C.; Merchán, A.V.; Galván, A.I.; Ruiz-Moyano, S. Bacterial communities in Serpa cheese by culture dependent techniques, 16S rRNA gene sequencing and high-throughput sequencing analysis. J. Food Sci. 2018, 83, 1333–1341. [Google Scholar] [CrossRef]
- Chaves-López, C.; De Angelis, M.; Martuscelli, M.; Serio, A.; Paparella, A.; Suzzi, G. Characterization of the Enterobacteriaceae isolated from an artisanal Italian ewe’s cheese (Pecorino Abruzzese). J. Appl. Microbiol. 2006, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Tabla, R.; Gómez, A.; Simancas, A.; Rebollo, J.E.; Molina, F.; Roa, I. Enterobacteriaceae species during manufacturing and ripening of semi–hard and soft raw ewe’s milk cheese: Gas production capacity. Small Rumin. Res. 2016, 145, 123–129. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Sampaio, P. Volatile fraction of DOP “Castelo Branco” cheese: Influence of breed. Food Chem. 2009, 112, 1053–1059. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Delgado, F.J.; Rodríguez-Pinilla, J.; González-Crespo, J.; Ramírez, R.; Roa, I. Proteolysis and texture changes of a Spanish soft cheese (‘Torta del Casar’) manufactured with raw ewe milk and vegetable rennet during ripening. Int. J. Food Sci. Technol. 2010, 45, 512–519. [Google Scholar] [CrossRef]
- Delgado-Martínez, F.J.; Carrapiso, A.I.; Contador, R.; Ramírez, M.R. Volatile compounds and sensory changes after high pressure processing of mature “Torta del Casar” (raw ewe’s milk cheese) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2019, 52, 34–41. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–211. [Google Scholar] [CrossRef]
- Calzada, J.; del Olmo, A.; Picon, A.; Nuñez, M. Effect of high pressure processing on the lipolysis, volatile compounds, odour and colour of cheese made from unpasteurized milk. Food Bioprocess Technol. 2015, 8, 1076–1088. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G. Identification of aroma compounds in Parmigiano-Reggiano cheese by gas chromatography/olfactometry. J. Dairy Sci. 2002, 85, 1362–1369. [Google Scholar] [CrossRef]
- Ghiaci, P.; Lameiras, F.; Norbeck, J.; Larsson, C. Production of 2-butanol through meso-2,3-butanediol consumption in lactic acid bacteria. FEMS Microbiol. Lett. 2014, 360, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Feliu, I.; Fernández-García, E.; Nuñez, M. Volatile compounds produced in cheese by Enterobacteriaceae strains of dairy origin. J. Food Prot. 2004, 67, 567–573. [Google Scholar] [CrossRef]
- Carbonell, M.; Nuñez, M.; Fernández-García, E. Evolution of the volatile components of ewe raw milk La Serena cheese during ripening. Correlation with flavour characteristics. Lait 2002, 82, 683–698. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME-GC-MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- van Mastrigt, O.; Gallegos Tejeda, D.; Kristensen, M.N.; Abee, T.; Smid, E.J. Aroma formation during cheese ripening is best resembled by Lactococcus lactis retentostat cultures. Microb. Cell Fact. 2018, 17, 104. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Meng, H.Y.; Piccand, M.; Fuchsmann, P.; Dubois, S.; Baumeyer, A.; Tena Stern, M.; Von Ah, U. Formation of 3-Methylbutanal and 3-Methylbutan-1-ol Recognized as Malty during Fermentation in Swiss Raclette-Type Cheese, Reconstituted Milk, and de Man, Rogosa, and Sharpe Broth. J. Agric. Food Chem. 2021, 69, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Castada, H.Z.; Hanas, K.; Barringer, S.A. Swiss cheese flavor variability based on correlations of volatile flavor compounds, descriptive sensory attributes, and consumer preference. Foods 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; Rodríguez-Pinilla, J.; Márquez, G.; Roa, I.; Ramírez, R. Physicochemical, proteolysis and texture changes during the storage of a mature soft cheese treated by high-pressure hydrostatic. Eur. Food Res. Technol. 2015, 240, 1167–1176. [Google Scholar] [CrossRef]
- Frank, O.; Jezussek, M.; Hofmann, T. Sensory activity, chemical structure, and synthesis of Maillard generated bitter-tasting 1-oxo-2,3-dihydro-1H-indolizinium-6-olates. J. Agric. Food Chem. 2003, 51, 2693–2699. [Google Scholar] [CrossRef]
- Medved’ová, A.; Koňuchová, M.; Kvočiková, K.; Hatalová, I.; Valík, L. Effect of lactic acid bacteria addition on the microbiological safety of Pasta-filata types of cheeses. Front. Microbiol. 2020, 11, 1–16. [Google Scholar] [CrossRef]



| Batches | Microorganism | Days of Ripening | |||
|---|---|---|---|---|---|
| 0 | 45 | 60 | 90 | ||
| C | TAM | 8.08 ± 0.034 cB | 8.29 ± 0.237 aAB | 8.36 ± 0.199 bA | 8.37 ± 0.065 aA |
| LAB | 8.14 ±0.079 aA | 8.11 ± 0.216 aA | 8.24 ± 0.159 aA | 8.32 ± 0.132 aA | |
| E | 6.81 ± 0.294 aA | 6.17 ± 0.293 aAB | 6.13 ± 0.245 aAB | 5.54 ± 0.629 aB | |
| Lc | TAM | 8.28 ± 0.046 bB | 8.62 ± 0.215 aB | 9.06 ± 0.283 aA | 8.33 ± 0.141 aB |
| LAB | 8.30 ± 0.193 aA | 8.55 ± 0.158 aA | 8.68 ± 0.399 aA | 8.14 ± 0.150 aA | |
| E | 6.74 ± 0.307 aA | 6.02 ± 0.182 aB | 5.81 ± 0.261 aB | 5.71 ± 0.323 aB | |
| Lg | TAM | 8.41 ± 0.050 aAB | 8.5 ± 0.192 aA | 8.53 ± 0.355 bA | 7.99 ± 0.316 bB |
| LAB | 8.23 ± 0.057 aA | 8.31 ± 0.293 aA | 8.50 ± 0.229 aA | 7.94 ± 0.314 aA | |
| E | 6.96 ± 0.144 aA | 6.13 ± 0.370 aB | 5.97 ± 0.097 aBC | 5.54 ± 0.255 aC | |
| Origin/Compound | Batches | Days of Ripening | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 45 | 60 | 90 | ||
| Acids | ||||||
| Acetic acid | C | 5.88 ± 1.114 bC | 14.44 ± 5.803 aA | 15.44 ± 3.855 aA | 16.88 ± 4.768 aA | 8.54 ± 0.059 aB |
| Lc | 10.50 ± 2.972 aBC | 16.94 ± 0.681 aA | 13.66 ± 1.638 aAB | 14.33 ± 3.647 aAB | 7.42 ± 0.678 aC | |
| Lg | 7.72 ± 1.183 bAB | 14.58 ± 2.117 aAB | 16.12 ± 6.524 aA | 18.90 ± 6.212 aA | 9.25 ± 0.356 aC | |
| Butanoic acid | C | 1.86 ± 0.429 aB | 3.00 ± 0.258 aB | 2.97 ± 0.406 bB | 2.99 ± 0.138 bB | 5.46 ± 1.380 aA |
| Lc | 1.88 ± 0.349 aB | 3.14 ± 0.143 aAB | 2.93 ± 0.450 bAB | 3.46 ± 0.649 bAB | 4.90 ± 1.446 aA | |
| Lg | 1.95 ± 0.615 aC | 3.01 ± 0.012 aB | 4.35 ± 0.439 aAB | 4.97 ± 0.497 aA | 5.45 ± 0.757 aA | |
| Hexanoic acid | C | 4.52 ± 0.937 aC | 4.44 ± 0.948 aC | 8.19 ± 0.618 ab2 | 9.67 ± 0.778 aAB | 11.32 ± 2.618 aA |
| Lc | 5.06 ± 1.212 aB | 7.86 ± 0.986 aAB | 8.83 ± 1.899 a12 | 8.83 ± 0.963 aAB | 11.68 ± 4.520 aA | |
| Lg | 5.81 ± 1.496 aB | 7.08 ± 0.789 aB | 6.84 ± 0.646 b2 | 9.75 ± 1.75 aA | 9.99 ± 1.534 aA | |
| Octanoic acid | C | 0.60 ± 0.148 bB | 0.38 ± 0.049 bB | 1.71 ± 0.313 aA | 2.13 ± 0.252 aA | 2.34 ± 0.692 aA |
| Lc | 0.99 ± 0.330 aB | 1.62 ± 0.466 aAB | 2.60 ± 1.631 aAB | 2.57 ± 0.714 aAB | 4.64 ± 3.324 aA | |
| Lg | 0.95 ± 0.255 aAB | 1.43 ± 0.362 aAB | 0.47 ± 0.246 aB | 1.62 ± 1.301 aAB | 1.94 ±0.493 aA | |
| Propanoic acid | C | n.d. | 0.28 ± 0.118 bB | 0.97 ± 0.446 aAB | 1.53 ± 0.440 aA | 0.72 ± 0.529 bAB |
| Lc | n.d. | 0.88 ± 0.560 aA | 1.47 ± 0.955 aA | 1.42 ± 0.467 aA | 1.45 ± 0.410 abA | |
| Lg | n.d. | 0.25 ± 0.074 bB | 0.41 ± 0.155 aB | 1.26 ± 0.412 aA | 1.71 ± 0.401 aA | |
| 2-methyl-propanoic acid | C | 0.12 ± 0.002 aC | 2.64 ± 1.918 aB | 6.01 ± 2.742 aA | 6.40 ± 2.368 aA | 3.45 ± 0.702 bB |
| Lc | 0.12 ± 0.002 aC | 3.87 ± 1.506 aB | 8.21 ± 1.757 aAB | 7.18 ± 2.259 aAB | 11.94 ± 3.156 aA | |
| Lg | 0.13 ± 0.004 aC | 1.02 ± 0.375 bC | 6.83 ± 1.688 aB | 6.65 ± 2.955 aB | 11.02 ± 4.210 aA | |
| 3-methyl-butanoic acid | C | 2.00 ± 0.265 aC | 23.93 ± 1.513 aB | 47.41 ± 5.618 aA | 54.71 ± 2.382 bA | 28.59 ± 4.543 bB |
| Lc | 0.69 ± 0.113 bD | 16.41 ± 2.777 aC | 41.59 ± 9.609 aBC | 126.91 ± 12.188 aA | 79.43 ± 3.102 aB | |
| Lg | 1.33 ± 0.973 abC | 10.13 ± 2.629 bB | 15.85 ± 2.838 bB | 63.26 ± 3.974 bA | 61.67 ± 2.608 aA | |
| 2-methyl-butanoic acid | C | n.d. | 0.93 ± 0.541 aB | 3.17 ± 1.271 aA | 3.78 ± 0.614 aA | 2.75 ± 0.631 bA |
| Lc | n.d. | 1.37 ± 0.874 aC | 2.36 ± 0.771 abBC | 5.08 ± 2.271 aA | 4.35 ± 0.768 aAB | |
| Lg | n.d. | 0.60 ± 0.145 aB | 1.04 ± 0.258 bB | 3.11 ± 1.435 aAB | 3.83 ± 0.749 abA | |
| 3-methyl-2-butenioc acid | C | n.d. | n.d. | 0.19 ± 0.040 aA | 0.29 ± 0.068 aA | 0.17 ± 0.041 aA |
| Lc | n.d. | n.d. | 0.22 ± 0.058 aA | 0.35 ± 0.172 aA | 0.20 ± 0.055 aA | |
| Lg | n.d. | n.d. | 0.14 ± 0.002 aA | 0.20 ± 0.078 aA | 0.16 ± 0.020 aA | |
| Origin/Compound | Batches | Days of Ripening | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 45 | 60 | 90 | ||
| Alcohols | ||||||
| 2-butanol, (R)- | C | n.d. | n.d. | 4.27 ± 1.14 bB | 12.42 ± 0.860 aA | 12.94 ± 1.005 aA |
| Lc | n.d. | n.d. | 7.58 ± 1.94 aA | 5.27 ± 1.198 bA | 5.26 ± 0.827 bA | |
| Lg | n.d. | n.d. | 2.21 ± 0.311 cB | 10.21 ± 3.759 aA | 12.70 ± 1.434 aA | |
| 2-methyl-1-propanol | C | 0.19 ± 0.014 aAB | 0.20 ± 0.011 aAB | 0.17 ± 0.031 aB | 0.14 ± 0.032 aB | 0.25 ± 0.081 aA |
| Lc | 0.18 ± 0.026 aA | 0.17 ± 0.027 aA | 0.16 ± 0.038 aA | 0.14 ± 0.035 aA | 0.14 ± 0.002 bA | |
| Lg | 0.17 ± 0.010 aA | 0.18 ± 0.052 aA | 0.16 ± 0.044 aA | 0.15 ± 0.058 aA | 0.17 ± 0.034 abA | |
| 3-methyl-1-butanol | C | 8.29 ± 0.538 aA | 5.70 ± 0.712 aB | 3.92 ± 0.426 aC | 3.20 ± 0.267 aC | 2.97 ± 0.607 aC |
| Lc | 6.14 ± 0.192 bA | 4.63 ± 0.755 aB | 4.09 ± 1.013 aB | 2.61 ± 0.804 aC | 2.57 ± 0.211 aC | |
| Lg | 5.93 ± 0.219 bA | 5.14 ± 0.410 aA | 3.94 ± 0.818 aB | 3.40 ± 0.890 aBC | 2.75 ± 0.286 aC | |
| 2-methyl-1-butanol | C | n.d. | 0.11 ± 0.016 aA | n.d. | n.d. | 0.16 ± 0.042 aA |
| Lc | n.d. | n.d. | n.d. | 0.12 ± 0.022 aA | 0.21 ± 0.015 aA | |
| Lg | n.d. | n.d. | 0.12 ± 0.009 aA | 0.11 ± 0.000 aA | 0.15 ± 0.027 aA | |
| Phenylethyl alcohol | C | 1.36 ± 0.137 aA | 0.86 ± 0.094 bC | 1.04 ± 0.151 aBC | 1.02 ± 0.173 aAB | 0.93 ± 0.089 aC |
| Lc | 1.25 ± 0.039 aA | 1.08 ± 0.075 aA | 1.07 ± 0.308 aA | 0.99 ± 0.145 aA | 0.89 ± 0.207 aA | |
| Lg | 1.30 ± 0.404 aA | 1.21 ± 0.180 aAB | 0.92 ± 0.164 aAB | 0.97 ± 0.149 aAB | 0.80 ± 0.149 aB | |
| 2,3-butanediol, [R-(R*,R*)]- | C | 2.80 ± 0.853 aB | 15.42 ± 1.458 aA | 10.01 ± 1.038 bA | 9.59 ± 1.204 abAB | 10.93 ± 0.449 aA |
| Lc | 2.54 ± 0.822 aB | 14.50 ± 1.181 aA | 4.20 ± 0.973 cAB | 6.19 ± 0.959 bAB | 3.09 ± 0.144 bB | |
| Lg | 2.58 ± 0.738 aC | 14.32 ± 1.674 aAB | 14.81 ± 0.634 aA | 12.59 ± 1.500 aA | 5.77 ± 0.528 bBC | |
| 2-butoxy-ethanol | C | n.d. | 0.27 ± 0.054 aA | 0.20 ± 0.089 bAB | 0.15 ± 0.021 bB | 0.23 ± 0.083 bAB |
| Lc | n.d. | 0.30 ± 0.056 aA | 0.24 ± 0.082 bAB | 0.20 ± 0.033 bB | 0.22 ± 0.039 bAB | |
| Lg | n.d. | 0.32 ± 0.052 aB | 0.39 ± 0.036 aA | 0.37 ± 0.041 aA | 0.36 ± 0.042 aA | |
| 2,6-dimethyl-4-heptanol | C | n.d. | 0.26 ± 0.004 aA | 0.37 ± 0.462 aA | 0.19 ± 0.022 aA | 0.18 ± 0.037 aA |
| Lc | n.d. | 0.19 ± 0.017 aB | 0.66 ± 0.146 aA | 0.28 ± 0.159 aB | 0.37 ± 0.029 aB | |
| Lg | n.d. | 0.24 ± 0.023 aA | 0.35 ± 0.211 aA | 0.18 ± 0.048 aA | 0.19 ± 0.015 aA | |
| Ketones | ||||||
| 2-nonanone | C | 0.38 ± 0.086 abB | 0.14 ± 0.038 aB | 0.41 ± 0.339 aB | 0.19 ± 0.002 aB | 0.61 ± 0.021 bA |
| Lc | 0.54 ± 0.182 aB | 0.13 ± 0.006 aB | 0.21 ± 0.089 aB | 0.18 ± 0.056 aB | 0.95 ± 0.050 aA | |
| Lg | 0.23 ± 0.028 bB | 0.16 ± 0.036 aB | 0.18 ± 0.051 aB | 0.11 ± 0.006 aB | 0.79 ± 0.152 abA | |
| 2-heptanone | C | 0.32 ± 0.009 aAB | 0.12 ± 0.032 aB | 0.26 ± 0.078 aAB | 0.16 ± 0.052 aB | 5.23 ± 2.880 aA |
| Lc | 0.38 ± 0.138 aAB | 0.12 ± 0.004 aB | 0.19 ± 0.091 aAB | 0.18 ± 0.060 aAB | 1.10 ± 0.096 bA | |
| Lg | 0.27 ± 0.057 aB | 0.13 ± 0.004 aB | 0.28 ± 0.155 aB | 0.13 ± 0.009 aB | 0.98 ± 0.147 bA | |
| 2,3-butanedione | C | 0.25 ± 0.040 aC | 0.57 ± 0.104 aABC | 0.88 ± 0.322 aA | 0.72 ± 0.221 aAB | 0.40 ± 0.115 abBC |
| Lc | 0.27 ± 0.066 aC | 0.43 ± 0.075 abC | 1.00 ± 0.128 aA | 0.69 ± 0.140 aB | 0.34 ± 0.080 bC | |
| Lg | 0.26 ± 0.079 aC | 0.39 ± 0.104 bBC | 1.16 ± 0.202 aA | 0.56 ± 0.066 aB | 0.41 ± 0.037 aBC | |
| 2-pentanone | C | 0.16 ± 0.038 aA | 0.12 ± 0.012 aA | 0.15 ± 0.004 aA | n.d. | 1.27 ± 0.103 aA |
| Lc | n.d. | n.d. | n.d. | n.d. | 0.87 ± 0.098 aA | |
| Lg | n.d. | n.d. | 0.16 ± 0.095 aA | n.d. | 0.40 ± 0.021 aA | |
| 2-butanone | C | 0.30 ± 0.099 aD | 1.12 ± 0.626 aD | 10.17 ± 1.942 aC | 18.70 ± 1.575 aB | 35.37 ± 4.673 aA |
| Lc | 0.31 ± 0.096 aD | 1.13 ± 0.403 aD | 9.62 ± 1.403 aC | 15.76 ± 1.897 bB | 25.99 ± 3.316 bA | |
| Lg | 0.28 ± 0.117 aD | 0.53 ± 0.220 aD | 9.30 ± 3.246 aC | 17.64 ± 1.161 abA | 31.63 ± 1.967 abA | |
| Aldehydes | ||||||
| 3-methyl-butanal | C | 0.17 ± 0.020 aB | 0.38 ± 0.112 aAB | 0.34 ± 0.142 abAB | 0.61 ± 0.202 aA | 0.60 ± 0.049 aAB |
| Lc | 0.17 ± 0.020 aB | 0.33 ± 0.151 aAB | 0.55 ± 0.100 aA | 0.41 ± 0.185 bAB | 0.69 ± 0.027 aA | |
| Lg | 0.16 ±0.013 aB | 0.22 ± 0.039 aB | 0.21 ± 0.088 bB | 0.31 ± 0.155 bB | 0.68 ± 0.130 aA | |
| Origin/Compound | Batches | Days of Ripening | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 45 | 60 | 90 | ||
| Esters | ||||||
| Butanoic acid, ethyl ester | C | 1.67 ± 0.156 aA | 0.40 ± 0.151 aB | 0.17 ± 0.027 aBC | 0.12 ± 0.003 aC | 0.14 ± 0.002 aC |
| Lc | 1.69 ± 0.058 aA | 0.24 ± 0.028 bB | 0.15 ± 0.024 aC | 0.18 ± 0.061 aBC | 0.13 ± 0.017 aC | |
| Lg | 2.00 ± 0.449 aA | 0.26 ± 0.089 abB | 0.21 ± 0.052 aB | 0.19 ± 0.046 aB | 0.15 ± 0.010 aB | |
| Hexanoic acid, ethyl ester | C | 2.57 ± 0.439 aA | 0.79 ± 0.233 aB | 0.54 ± 0.069 abB | 0.43 ± 0.042 aB | 0.53 ± 0.090 aB |
| Lc | 2.08 ± 0.057 aA | 0.57 ± 0.058 aB | 0.40 ± 0.067 bC | 0.46 ± 0.148 aBC | 0.32 ± 0.032 bC | |
| Lg | 2.61 ± 0.856 aA | 0.69 ± 0.130 aB | 0.66 ± 0.158 aB | 0.58 ± 0.113 aB | 0.56 ± 0.050 aB | |
| Octanoic acid, ethyl ester | C | 1.85 ± 0.305 aA | 0.40 ± 0.087 aB | 0.41 ± 0.030 aB | 0.41 ± 0.018 aB | 0.49 ± 0.053 aB |
| Lc | 1.60 ± 0.134 aA | 0.42 ± 0.044 aB | 0.40 ± 0.041 aB | 0.35 ± 0.040 bB | 0.35 ± 0.028 bB | |
| Lg | 1.97 ± 0.757 aA | 0.42 ± 0.038 aB | 0.36 ± 0.055 aB | 0.36 ± 0.032 abB | 0.45 ± 0.040 aB | |
| Decanoic acid, ethyl ester | C | 2.18 ± 0.258 bA | 0.56 ± 0.135 aB | 0.62 ± 0.053 aB | 0.65 ± 0.030 aB | 0.64 ± 0.073 aB |
| Lc | 2.79 ± 0.577 abA | 0.65 ± 0.075 aB | 0.68 ± 0.102 aB | 0.53 ± 0.044 bB | 0.45 ± 0.094 bB | |
| Lg | 2.97 ± 0.856 aA | 0.62 ± 0.065 aB | 0.48 ± 0.058 bB | 0.52 ± 0.051 bB | 0.56 ± 0.111 abB | |
| Dodecanoic acid, ethyl ester | C | 0.18 ± 0.025 a | n.d. | n.d. | n.d. | n.d. |
| Lc | 0.21 ± 0.044 a | n.d. | n.d. | n.d. | n.d. | |
| Lg | 0.24 ± 0.069 a | n.d. | n.d. | n.d. | n.d. | |
| 1-butanol, 3-methyl-, acetate | C | 0.37 ± 0.015 aA | 0.37 ± 0.035 aA | 0.28 ± 0.043 abAB | 0.23 ± 0.042 aB | 0.33 ± 0.093 aAB |
| Lc | 0.35 ± 0.039 aA | 0.27 ± 0.065 bAB | 0.18 ± 0.025 bB | 0.19 ± 0.095 aB | 0.19 ± 0.025 bB | |
| Lg | 0.37 ± 0.027 aA | 0.27 ± 0.046 bA | 0.34 ± 0.087 aA | 0.30 ± 0.099 aA | 0.35 ± 0.025 aA | |
| Others compounds | ||||||
| 1,5,9-decatriene, 2,3,5,8-tetramethyl- | C | 0.60 ± 0.089 bC | 0.61 ± 0.108 bC | 0.77 ± 0.048 abAB | 0.89 ± 0.025 aA | 0.67 ± 0.023 aBC |
| Lc | 0.71 ± 0.084 abAB | 0.85 ± 0.068 aA | 0.85 ± 0.073 aA | 0.70 ± 0.051 bAB | 0.64 ± 0.133 aB | |
| Lg | 0.76 ± 0.017 aAB | 0.80 ± 0.053 aA | 0.68 ± 0.032 bB | 0.74 ± 0.048 bAB | 0.66 ± 0.089 aB | |
| Dimethyl ether | C | 29.18 ± 3.598 aA | 5.55 ± 3.239 aB | 1.39 ± 0.562 aBC | 1.18 ± 0.068 abBC | 0.66 ± 0.278 bBC |
| Lc | 31.91 ± 1.945 aA | 3.78 ± 0.655 aB | 1.30 ± 0.699 aC | 0.41 ± 0.075 bC | 0.25 ± 0.093 cC | |
| Lg | 31.42 ± 0.338 aA | 3.50 ± 1.693 aB | 1.63 ± 0.826 aC | 1.75 ± 0.689 aBC | 1.50 ± 0.080 aC | |
| Trimethyl-pyrazine | C | n.d. | n.d. | 0.13 ± 0.009 B | 0.14 ± 0.012 B | 0.42 ± 0.129 abA |
| Lc | n.d. | n.d. | n.d. | n.d. | 0.68 ± 0.099 a | |
| Lg | n.d. | n.d. | n.d. | n.d. | 0.28 ± 0.081 b | |
| 2,5-dimethyl-pyrazine | C | n.d. | n.d. | n.d. | n.d. | 0.18 ± 0.007 a |
| Lc | n.d. | n.d. | n.d. | n.d. | 0.37 ± 0.054 a | |
| Lg | n.d. | n.d. | n.d. | n.d. | 0.15 ± 0.008 a | |
| Dimethyl disulfide | C | 0.15 ± 0.020 aB | 0.52 ± 0.085 aA | 0.36 ± 0.094 aAB | 0.21 ± 0.038 aB | 0.17 ± 0.006 aB |
| Lc | n.d. | 0.50 ± 0.089 aA | 0.35 ± 0.011 aAB | 0.29 ± 0.022 aB | 0.16 ± 0.023 aB | |
| Lg | n.d. | 0.27 ± 0.044 bA | 0.16 ± 0.039 bB | 0.28 ± 0.082 aA | 0.14 ± 0.002 aB | |
| Parameters | Batches | ||
|---|---|---|---|
| C | Lc | Lg | |
| Hardness (N) | 5.05 ± 2.663 | 5.18 ± 1.579 | 4.44 ± 1.702 |
| Adhesiveness (N/s) | −0.36 ± 0.192 | −0.55 ± 0.312 | −0.47 ± 0.262 |
| Springiness | 0.74 ± 0.052 | 0.76 ± 0.068 | 0.75 ± 0.079 |
| Cohesiveness | 0.64 ± 0.049 | 0.64 ± 0.033 | 0.65 ± 0.039 |
| Chewiness (N) | 2.66 ± 0.831 | 2.57 ± 0.885 | 2.54 ± 0.828 |
| CIE L* | 101.09 ± 6.250 | 100.51 ± 2.730 | 97.11 ± 3.740 * |
| CIE a* | −1.43 ± 0.859 | −1.26 ± 0.920 | −1.72 ± 0.640 |
| CIE b* | 5.49 ± 2.510 | 6.26 ± 1.190 | 5.17 ± 2.211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, I.; Rodríguez, A.; García, C.; Córdoba, J.J. Evolution of Volatile Compounds during Ripening and Final Sensory Changes of Traditional Raw Ewe’s Milk Cheese “Torta del Casar” Maturated with Selected Protective Lactic Acid Bacteria. Foods 2022, 11, 2658. https://doi.org/10.3390/foods11172658
Martín I, Rodríguez A, García C, Córdoba JJ. Evolution of Volatile Compounds during Ripening and Final Sensory Changes of Traditional Raw Ewe’s Milk Cheese “Torta del Casar” Maturated with Selected Protective Lactic Acid Bacteria. Foods. 2022; 11(17):2658. https://doi.org/10.3390/foods11172658
Chicago/Turabian StyleMartín, Irene, Alicia Rodríguez, Carmen García, and Juan J. Córdoba. 2022. "Evolution of Volatile Compounds during Ripening and Final Sensory Changes of Traditional Raw Ewe’s Milk Cheese “Torta del Casar” Maturated with Selected Protective Lactic Acid Bacteria" Foods 11, no. 17: 2658. https://doi.org/10.3390/foods11172658
APA StyleMartín, I., Rodríguez, A., García, C., & Córdoba, J. J. (2022). Evolution of Volatile Compounds during Ripening and Final Sensory Changes of Traditional Raw Ewe’s Milk Cheese “Torta del Casar” Maturated with Selected Protective Lactic Acid Bacteria. Foods, 11(17), 2658. https://doi.org/10.3390/foods11172658