High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether
Abstract
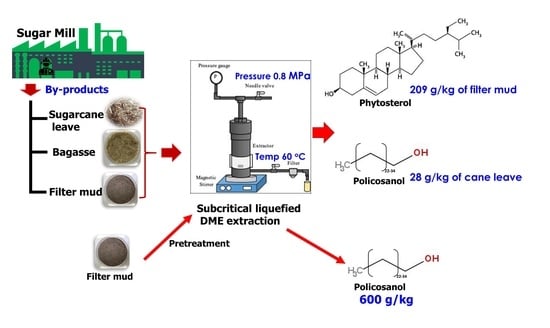
:1. Introduction
2. Materials and Methods
2.1. Materials and Standard Reagents
2.2. Solvent Extraction of Crude Extract from Samples
2.3. Extraction from By-Product Samples by SUBLE Using DME
2.4. Policosanol Analysis
2.4.1. Policosanol Derivatization
2.4.2. Gas Chromatography–Mass Spectrometry (GC-MS) Conditions for Policosanol Analysis
2.5. Analysis of Phytosterols
2.5.1. Extraction of Phytosterols
2.5.2. Phytosterol Derivatizations
2.5.3. Quantification and Identification of Phytosterols by GC-MS
2.6. Sample Pretreatment before SUBLDME Extraction on Policosanol and Phytosterol Contents
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Particle Size and Extraction Temperature on Yield and Phytosterol Content by SUBLDME Extraction
3.2. SUBLDME Extraction of Policosanols and Phytosterols Compared with Solvent on Soxhlet Extraction
3.3. Effect of Sample Pretreatment following SUBLDME Extraction on the Yield and Policosanol and Phytosterol Contents of Filter Mud
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Subcritical liquefied dimethyl ether | (SUBLDME) |
| Subcritical liquefied extraction | (SUBLE) |
| Supercritical fluid extraction | (SFE) |
| Dimethyl ether | (DME) |
| Trimethylsilyl | (TMS) |
| Supercritical fluid CO2 extraction | (SF-CO2) |
| Global warming potential | (GWP) |
References
- FAOSTAT. Food, Agricultural Organization of the United Nations Statistical Database; Agriculture Data; FAOSTAT: Rome, Italy, 2001. [Google Scholar]
- Eggleston, G.; Klich, M.; Antoine, A.; Beltz, S.; Viator, R. Brown and green sugarcane leaves as potential biomass: How they deteriorate under dry and wet storage conditions. Ind. Crops Prod. 2014, 57, 69–81. [Google Scholar] [CrossRef]
- Singh, A.; Lal, U.R.; Mukhtar, H.M.; Singh, P.S.; Shah, G.; Dhawan, R.K. Phytochemical profile of sugarcane and its potential health aspects. Pharmacogn. Rev. 2015, 9, 45–54. [Google Scholar] [CrossRef]
- Gupta, A.K.; Savopoulos, C.G.; Ahuja, J.; Hatzitolios, A.I. Role of phytosterols in lipid-lowering: Current perspectives. QJM Int. J. Med. 2011, 104, 301–308. [Google Scholar] [CrossRef]
- Rapport, L.; Lockwood, B. Nutraceuticals: (3) Octacosanol. Pharm. J. 2000, 265, 170–171. [Google Scholar]
- Shimura, S.; Hasegawa, T.; Takano, S.; Suzuki, T. Studies on the effect of octacosanol on motor endurance in mice. Nutr. Rep. Int. 1987, 36, 1029–1038. [Google Scholar]
- Lukashevich, V.; Davidson, M.H.; Moreines, J.; Berlin, R.G. Beeswax policosanol failed to demonstrate lipid-altering effects in well-controlled clinical trials. Circulation 2006, 114, 892. [Google Scholar]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Can, A.; Tchuenbou-Magaia, F.L. Development and characterization of phytosterol-enriched oil microcapsules for foodstuff application. Food Bioprocess Technol. 2018, 11, 152–163. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Feng, S.; Liu, S.; Luo, Z.; Tang, K. Direct saponification preparation and analysis of free and conjugated phytosterols in sugarcane (Saccharum officinarum L.) by reversed-phase high-performance liquid chromatography. Food Chem. 2015, 181, 9–14. [Google Scholar] [CrossRef]
- Feng, S.; Luo, Z.; Zhang, Y.; Zhong, Z.; Lu, B. Phytochemical contents and antioxidant capacities of different parts of two sugarcane (Saccharum officinarum L.) cultivars. Food Chem. 2014, 151, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.; Omar, A. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Attard, T.M.; McElroy, C.R.; Rezende, C.A.; Polikarpov, I.; Clark, J.H.; Hunt, A.J. Sugarcane waste as a valuable source of lipophilic molecules. Ind. Crops Prod. 2015, 76, 95–103. [Google Scholar] [CrossRef]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and recovery methods. Bioresour. Technol. 2007, 98, 2335–2350. [Google Scholar] [CrossRef]
- Alvarez-Vinas, M.; Rodríguez-Seoane, P.; Flórez-Fernández, N.; Torres, M.D.; Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Subcritical water for the extraction and hydrolysis of protein and other fractions in biorefineries from agro-food wastes and algae: A Review. Food Bioprocess Technol. 2021, 14, 373–387. [Google Scholar] [CrossRef]
- Guo, T.; Wan, C.; Huang, F. Extraction of rapeseed cake oil using subcritical R134a/butane: Process optimization and quality evaluation. Food Sci. Nutr. 2019, 7, 3570–3580. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ghazani, S.M.; Corazza, M.L.; Marangoni, A.G.; Ribani, R.H. Assessment of subcritical propane, supercritical CO2 and Soxhlet extraction of oil from sapucaia (Lecythis pisonis) nuts. J. Supercrit. Fluids 2018, 133, 122–132. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the safety of use of dimethyl ether as an extraction solvent under the intended conditions of use and the proposed maximum residual limits. EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF). EFSA J. 2015, 13, 4174. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Tallon, S.J.; Grey, J.B.; Fenton, K.; Fletcher, K.; Fletcher, A.J. Extraction of lipids from aqueous protein-rich streams using near-critical dimethyl ether. Chem. Eng. Technol. 2007, 30, 501–510. [Google Scholar] [CrossRef]
- Subratti, A.; Lalgee, L.J.; Jalsa, N.K. Liquified dimethyl ether (DME): A green solvent for the extraction of hemp (Cannabis sativa L.) seed oil. Sustain. Chem. Pharm. 2019, 12, 100144. [Google Scholar] [CrossRef]
- Subratti, A.; Lalgee, L.J.; Jalsa, N.K. Efficient extraction of black cumin (Nigella sativa L.) seed oil containing thymol, using liquefied dimethyl ether (DME). J. Food Process. Preserv. 2019, 43, e13913. [Google Scholar] [CrossRef]
- Boonnoun, P.; Kurita, Y.; Kamo, Y.; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Wet extraction of lipids and astaxanthin from Haematococcus pluvialis by liquefied dimethyl ether. Nutr. Food Sci. 2014, 4, 305. [Google Scholar] [CrossRef]
- Boonnoun, P.; Shotipruk, A.; Kanda, H.; Goto, M. Optimization of rubber seed oil extraction using liquefied dimethyl ether. Chem. Eng. Commun. 2019, 206, 746–753. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, S.; Liu, S.; Zhang, J.; Ding, Y.; Liu, J. Extraction of oil from high-moisture tuna liver by subcritical dimethyl ether: Feasibility and optimization by the response surface method. RSC Adv. 2018, 8, 2723–2732. [Google Scholar] [CrossRef]
- Furukawa, H.; Kikuchi, A.; Noriyasu, A.; Bouteau, F.; Nishihama, S.; Yoshizuka, K.; Li, X.; Kawano, T. Use of liquefied dimethyl ether for the extraction of proteins from vegetable tissues. Solvent Extr. Res. Dev. 2016, 23, 127–135. [Google Scholar] [CrossRef]
- Hoshino, R.; Machmudah, S.; Kanda, H.; Goto, M. Simultaneous extraction of water and essential oils from citrus leaves and peels using liquefied dimethyl ether. Nutr. Food Sci. 2014, 4, 5. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- Wongwaiwech, D.; Weerawatanakorn, M.; Boonnoun, P. Subcritical dimethyl ether extraction as a simple method to extract nutraceuticals from byproducts from rice bran oil manufacture. Sci. Rep. 2020, 10, 21007. [Google Scholar] [CrossRef]
- Asikin, Y.; Chinen, T.; Takara, K.; Wada, K. Determination of long-chain alcohol and aldehyde contents in the non-centrifuged cane sugar Kokuto. Food Sci. Technol. Res. 2008, 14, 583. [Google Scholar] [CrossRef]
- Wongwaiwech, D.; Weerawatanakorn, M.; Tharatha, S.; Ho, C.T. Comparative study on amount of nutraceuticals in by-products from solvent and cold pressing methods of rice bran oil processing. J. Food Drug Anal. 2019, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.H.J.; Li, T.S.C.; Drover, J.C. Phytosterol content in American ginseng seed oil. J. Agric. Food Chem. 2002, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.T.; Vergnes, M.F.; Kaloustian, J.; El-Moselhy, T.F.; Amiot-Carlin, M.J.; Portugal, H. Effect of storage and heating on phytosterol concentrations in vegetable oils determined by GC/MS. J. Sci. Food Agric. 2006, 86, 220–225. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef] [PubMed]
- Yatma, F.; Falah, S.; Ambarsari, L.; Aisyah, S.I.; Nurcholis, W. Optimization of extraction of phenolic and antioxidant activities from Celosia cristata seeds using response surface methodology. Biointerface Res. Appl. Chem. 2022, 13, 2069–5837. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Yunus, M.; Hasan, M.; Othman, N.; Mohd-Septapar, S.; Ahmad-Zaini, M.; Idham, Z.; Zhari, S. Effect of particle size on the oil yield and catechin compound using accelerated solvent extraction. J. Teknol. 2013, 60, 21–25. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.B.; Hazwan Ruslan, M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and Soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Patel, S.B.; Attar, U.A.; Sakate, D.M.; Ghane, S.G. Efficient extraction of cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: Optimization using response surface methodology, extraction methods and study of some important bioactivities. Sci. Rep. 2020, 10, 2109. [Google Scholar] [CrossRef]
- Zhao, S.; Kwok, K.C.; Liang, H. Investigation on ultrasound assisted extraction of saikosaponins from Radix bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, X.; Chen, X.D. Investigation on the relationship between the integrity of food matrix and nutrient extraction yield of broccoli. LWT—Food Sci. Technol. 2017, 85, 170–174. [Google Scholar] [CrossRef]
- Crossley, J.I.; Aguilera, J.M. Modeling the effect of microstructure on food extraction. J. Food Process. Eng. 2001, 24, 161–177. [Google Scholar] [CrossRef]
- Bao, N.; Rashed, M.M.; Jiang, B.; Zhai, K.; Luo, Z. Green and efficient extraction approach for polyphenol recovery from lotus seedpods (Receptaculum Nelumbinis): Gas-assisted combined with glycerol. ACS Omega 2021, 6, 26722–26731. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Takahashi, M.; Hirose, N.; Hou, D.X.; Takara, K.; Wada, K. Wax, policosanol, and long-chain aldehydes of different sugarcane (Saccharum officinarum L.) cultivars. Eur. J. Lipid Sci. Technol. 2012, 114, 583–591. [Google Scholar] [CrossRef]
- de Lucas, A.; García, A.; Alvarez, A.; Gracia, I. Supercritical extraction of long chain n-alcohols from sugar cane crude wax. J. Supercrit. Fluids. 2007, 41, 267–271. [Google Scholar] [CrossRef]
- Ou, S.; Zhao, J.; Wang, Y.; Tian, Y.; Wang, J. Preparation of octacosanol from filter mud produced after sugarcane juice clarification. LWT—Food Sci. Technol. 2012, 45, 295–298. [Google Scholar] [CrossRef]
- Sun, D.; Cao, C.; Li, B.; Chen, H.; Li, J.; Cao, P.; Liu, Y. Antarctic krill lipid extracted by subcritical n-butane and comparison with supercritical CO2 and conventional solvent extraction. LWT—Food Sci. Technol. 2018, 94, 1–7. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ohno, Y.; Goto, S.; Kajitani, S.; Konno, M.; Shikada, T.; Suzuki, S. DME Handbook; Japan DME Forum: Tokyo, Japan, 2007. [Google Scholar]
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct synthesis of dimethyl ether from CO2: Recent advances in bifunctional/hybrid catalytic systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Prado, J.M. Natural Product Extraction: Principles and Applications; Royal Society of Chemistry: London, UK, 2013; pp. 197–213. [Google Scholar]
- Eltringham, W.; Tallon, S.J.; Catchpole, O.J.; Fenton, K. Relative permittivity measurements of dimethyl ether + carbon dioxide mixtures. J. Chem. Eng. Data 2008, 53, 826–829. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Grey, J.B.; Noermark, K.A. Solubility of fish oil components in supercritical CO2 and CO2 + ethanol mixtures. J. Chem. Eng. Data 1998, 43, 1091–1095. [Google Scholar] [CrossRef]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Man, Y.B.C. Optimization of supercritical CO2 extraction of phytosterol-enriched oil from Kalahari melon seeds. Food Bioprocess Technol. 2011, 2, 1432–1441. [Google Scholar] [CrossRef]
- Georges, P.; Sylvestre, M.; Ruegger, H.; Bourgeois, P. Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids 2006, 71, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Jose, C.; Paulo, S.; Luiz, P.; Luciene, L.; Paulo, A.; Luiz, A.M.; Marcos, A.A.; Antonella, Z.; Alfesio, B. The impact of sugar cane–burning emissions on the respiratory system of children and the elderly. Environ. Health Perspect. 2006, 114, 725–729. [Google Scholar] [CrossRef]
- Casas, L.; Hernández, Y.; Mantell, C.; Casdelo, N.; Martinez de la Ossa, E. Filter cake oil-wax as raw material for the production of biodiesel: Analysis of the extraction process and the transesterification reaction. J. Chem. 2015, 2015, 946462. [Google Scholar] [CrossRef]
- Dunford, N.T.; Irmak, S.; Jonnala, R. Effect of the solvent type and temperature on phytosterol contents and compositions of wheat straw, bran, and germ extracts. J. Agric. Food Chem. 2009, 57, 10608–10611. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, M.J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Shaarani, S.; Sarker, M.Z.I. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Deng, C. Advance on the preparation technology and anti-hyperlipidemia mechanism of phytosterols. IOP Conf. Ser. Earth Environ. Sci. 2020, 615, 012107. [Google Scholar] [CrossRef]





| Conditions | Ultrasonic Treatment with Hexane | Subcritical Liquefied DME | Transesterification | Subcritical Liquefied DME |
|---|---|---|---|---|
| 1 | - |  | - | - |
| 2 |  |  | - | - |
| 3 |  |  |  | - |
| 4 |  |  |  |  |
 = The used process in each condition.
= The used process in each condition.| Size/Temp (Mesh/°C) | %Yield | Phytosterol Contents (mg/100 g) | Total | |||
|---|---|---|---|---|---|---|
| Campesterol | Stigmasterol | Beta-Sitosterol | Sitostanol | |||
| 40/35 | 2.14 ± 0.17 e | 884.49 ± 30.60 b | 507.54 ± 2.11 b | 787.80 ± 27.78 e | 145.84 ± 23.70 c | 2325.69 ± 32.58 d |
| 40/60 | 2.32 ± 0.76 b | 1381.7 ± 22.34 a, b | 689.00 ± 35.83 b | 1252.54 ± 3.52 d | 286.49 ± 40.15 b, c | 3609.73 ± 21.54 c |
| 60/35 | 2.04 ± 0.37 f | 1576.28 ± 3.60 a, b | 883.82 ± 6.24 a, b | 1376.51 ± 22.33 c, d | 373.53 ± 28.03 a, b | 4210.14 ± 60.21 b, c |
| 60/60 | 2.18 ± 0.14 d | 1619.47 ± 773.40 a, b | 1513.24 ± 563.18 a | 1931.06 ± 264.90 b | 340.47 ± 121.22 a, b | 5404.25 ± 826.73 a |
| 100/35 | 2.28 ± 0.27 c | 659.17 ± 15.68 b | 1655.97 ± 28.01 a | 2492.13 ± 16.84 a | 299.68 ± 0.34 a, b, c | 5106.96 ± 4.16 a, b |
| 100/60 | 2.68 ± 0.38 a | 2113.67 ± 19.38 a | 1180.95 ± 8.38 a, b | 1707.73 ± 61.18 b, c | 474.25 ± 43.85 a | 5476.70 ± 132.78 a |
| Soxhlet Extraction | SUBFE with DME Extraction | |||||
|---|---|---|---|---|---|---|
| Bagasse | Leaves | Filter Mud | Bagasse | Leaves | Filter Mud | |
| % Yield | 3.49 ± 0.08 b | 3.59 ± 0.32 a | 1.22 ± 0.31 d | 2.95 ± 0.12 c | 7.04 ± 0.22 a | 4.26 ± 0.02 b |
| Policosanol contents (mg/100 g) | ||||||
| C22 | 3.70 ± 0.11 d, A | 1.97 ± 0.08 e, B | 0.90 ± 0.25 e, C | 7.82 ± 0.26 c, B | 9.45 ± 1.36 b, B | 16.87 ± 0.20 a, A |
| C24 | 12.83 ± 0.21 c, A | 1.16 ± 0.20 d, B | 0.15 ± 0.04 d, C | 20.52 ± 2.40 b, B | 35.25 ± 0.16 a, A | 23.85 ± 5.24 b, B |
| C26 | 14.05 ± 0.03 c, A | 0.63 ± 0.16 e, B | 1.40 ± 0.39 e, B | 129.41 ± 1.77 a, A | 113.43 ± 0.08 b, B | 8.47 ± 2.38 d, C |
| C28 | 163.54 ± 16.21 d, A | 12.43 ± 1.26 e, B | 30.94 ± 8.60 e, B | 1737.10 ± 2.42 b, B | 2072.96 ± 1.08 a, A | 249.21 ± 1.39 c, C |
| C30 | 18.36 ± 1.19 d, A | 13.26 ± 3.47 e, A | 5.28 ± 1.46 f, B | 328.79 ± 0.82 b, B | 411.89 ± 0.82 a, A | 102.72 ± 8.03 c, C |
| C32 | 91.32 ± 0.54 d, A | 32.32 ± 0.12 e, B | 4.53 ± 1.25 f, C | 184.07 ± 3.71 b, B | 177.14 ± 0.28 c, B | 238.28 ± 4.85 a, A |
| C34 | 50.32 ± 0.47 a, A | 10.77 ± 3.10 d, B | 0.60 ± 0.17 e, C | 39.11 ± 2.71 b, A | 25.61 ± 2.17 c, B | 29.50 ± 2.22 c, B |
| Total | 354.12 ± 16.33 d, A | 72.54 ± 8.14 e, B | 43.78 ± 12.16 e, B | 2446.82 ± 14.07 b, B | 2845.71 ± 1.45 a, A | 668.90 ± 19.57 c, C |
| Phytosterol contents (mg/100 g) | ||||||
| Campesterol | 8.70 ± 1.25 d, B | 11.12 ± 3.01 d, B | 37.17 ± 1.92 d, A | 3788.98 ± 17.62 b, B | 966.83 ± 33.94 c, C | 4482.19 ± 1.84 a, A |
| Stigmasterol | 18.16 ± 0.02 d, C | 35.00 ± 3.83 d, B | 101.32 ± 0.06 d, A | 7234.98 ± 37.46 a, A | 4014.32 ± 112.47 c, C | 4423.49 ± 14.95 b, B |
| Beta-sitosterol | 47.68 ± 1.86 e, C | 65.00 ± 6.78 e, B | 137.18 ± 1.24 d, A | 8078.10 ± 46.41 b, B | 4848.16 ± 11.71 c, C | 11,804.11 ± 7.09 a, A |
| Sitostanol | 4.16 ± 0.31 d, B | 14.69 ± 3.56 d, A | 11.70 ± 0.75 d, A | 454.28 ± 2.89 a, A | 318.24 ± 21.30 b, B | 168.92 ± 6.48 c, C |
| Total | 78.67 ± 0.28 e, C | 125.81 ± 11.15 e, B | 287.36 ± 4.00 d, A | 19,556.33 ± 104.38 b, B | 10,147.55 ± 113.41 c, C | 20,878.75 ± 17.41 a, A |
| Extraction Conditions | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Percent yield of crude extract (based on filter mud) | 4.19 ± 0.12 a | 4.75 ± 0.56 a | 2.25 ± 0.02 b | 1.55 ± 0.01 c |
| Policosanol contents (mg/100 g) | ||||
| C22 | 16.87 ± 0.24 c | 64.92 ± 2.81 b | 22.17 ± 1.01 c | 164.66 ± 4.30 a |
| C24 | 23.85 ± 5.24 c | 810.25 ± 49.74 a | 74.73 ± 0.24 c | 511.65 ± 12.61 b |
| C26 | 8.47 ± 2.38 d | 1230.39 ± 35.64 b | 424.72 ± 7.31 c | 4103.30 ± 30.43 a |
| C28 | 249.21 ± 1.40 d | 1430.11 ± 44.18 c | 2880.63 ± 19.29 b | 29,726.90 ± 62.85 a |
| C30 | 102.72 ± 8.04 d | 1485.90 ± 24.44 c | 1897.28 ± 50.91 b | 17,233.82 ± 25.65 a |
| C32 | 238.28 ± 4.85 c | 307.81 ± 0.69 c | 812.58 ± 20.74 b | 7167.79 ± 51.87 a |
| C34 | 29.50 ± 2.22 d | 260.48 ± 5.39 b | 204.90 ± 5.21 c | 1148.10 ± 25.87 a |
| Total | 668.90 ± 10.92 d | 5589.87 ± 56.76 c | 6317.00 ± 77.16 b | 60,056.23 ± 73.50 a |
| Phytosterol contents (mg/100 g) | ||||
| Campesterol | 4482.19 ± 1.84 b | 4618.66 ± 36.82 a | 1600.01 ± 48.20 d | 3027.53 ± 7.87 c |
| Stigmasterol | 4423.49 ± 14.95 a | 3651.58 ± 13.06 b | 1201.00 ± 17.27 d | 2215.50 ± 2.60 c |
| Beta-sitosterol | 11,804.10 ± 7.09 a | 4478.10 ± 41.37 b | 1572.03 ± 11.44 d | 3020.79 ± 8.27 c |
| Sitostanol | 168.98 ± 6.47 d | 546.74 ± 16.15 b | 282.86 ± 20.82 c | 1734.35 ± 2.65 a |
| Total | 20,878.75 ± 17.41 a | 13,295.09 ± 24.67 b | 4655.90 ± 97.74 d | 9998.18 ± 16.09 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamchonemenukool, S.; Ho, C.-T.; Boonnoun, P.; Li, S.; Pan, M.-H.; Klangpetch, W.; Weerawatanakorn, M. High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether. Foods 2022, 11, 2937. https://doi.org/10.3390/foods11192937
Kamchonemenukool S, Ho C-T, Boonnoun P, Li S, Pan M-H, Klangpetch W, Weerawatanakorn M. High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether. Foods. 2022; 11(19):2937. https://doi.org/10.3390/foods11192937
Chicago/Turabian StyleKamchonemenukool, Sudthida, Chi-Tang Ho, Panatpong Boonnoun, Shiming Li, Min-Hsiung Pan, Wannaporn Klangpetch, and Monthana Weerawatanakorn. 2022. "High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether" Foods 11, no. 19: 2937. https://doi.org/10.3390/foods11192937
APA StyleKamchonemenukool, S., Ho, C.-T., Boonnoun, P., Li, S., Pan, M.-H., Klangpetch, W., & Weerawatanakorn, M. (2022). High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether. Foods, 11(19), 2937. https://doi.org/10.3390/foods11192937