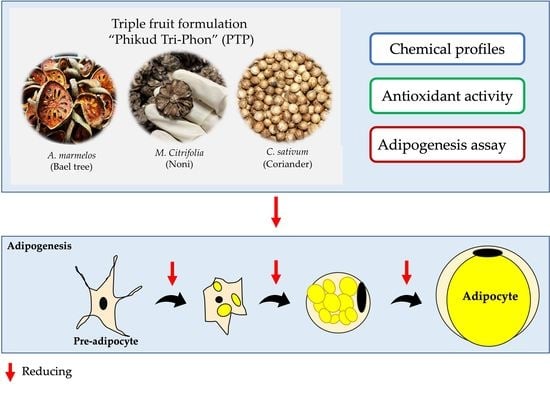
A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction of Phikud Tri-Phon (PTP) and the 3 Herbal Products
2.4. Determination of Total Phenolic Contents
2.5. Determination of Total Flavonoid Contents
2.6. Free Radical Scavenging Using DPPH
2.7. Cell Culture and Reagents
2.8. Cytotoxicity of Extracts on 3T3-L1 Pre-Adipocytes
2.9. Adipogenesis
2.9.1. Inhibition of Adipogenesis
2.9.2. Adipocyte Lipolysis
2.10. Quantification of Cellular Lipid Contents
2.11. Chemical Profiling of PTP by LC/MS
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Contents
3.2. Total Flavonoid Contents
3.3. DPPH Radical Scavenging
3.4. Cell Viability
3.5. The Extracts Reduce Adipogenesis
3.5.1. Effect on Lipid Accumulation
3.5.2. Lipolytic Actions
3.6. Structural Elucidation of PTP by LC/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2022).
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrients 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.; Mozaffarian, D. The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Lee, H.-W.; Rhee, D.-K.; Kim, B.-O.; Pyo, S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed. Pharmacother. 2018, 102, 670–680. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.; Qu, X. Comparison of Cytotoxicity and the Anti-Adipogenic Effect of Green Tea Polyphenols with Epigallocatechin-3-Gallate in 3T3-L1 Preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Tungkwampian, W.; Theerarungchaisri, A.; Buranarach, M. Development Thai herbal medicine knowledge base using ontology technique. Thai. J. Pharm Sci. 2015, 39, 102–109. [Google Scholar]
- Nalinratana, N.; Kaewprem, W.; Tongumpai, S.; Luechapudiporn, R.; Sotanaphun, U.; Meksuriyen, D. Synergistic antioxidant action of Phikud Navakot ameliorates hydrogen peroxide-induced stress in human endothelial cells. Integr. Med. Res. 2014, 3, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Karmase, A.; Birari, R.; Bhutani, K.K. Evaluation of anti-obesity effect of Aegle marmelos leaves. Phytomedicine 2013, 20, 805–812. [Google Scholar] [CrossRef]
- Karmase, A.; Jagtap, S.; Bhutani, K.K. Anti adipogenic activity of Aegle marmelos Correa. Phytomedicine 2013, 20, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, V.K. Anti-Fertility Role of Aegle Marmelos (Bael). J. Appl. Health Sci. Med. 2022, 2, 21–25. [Google Scholar]
- Agrawal, S.S.; Kumar, A.; Gullaiya, S.; Dubey, V.; Nagar, A.; Tiwari, P.; Dhar, P.; Singh, V. Antifertility activity of methanolic bark extract of Aegle marmelos (L.) in male wistar rats. DARU J. Pharm. Sci. 2012, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Agarwal, M.; Kushwaha, S.; Mutreja, A. Suppression of fertility in male albino rats following the administration of 50% ethanolic extract of Aegle marmelos. Contraception 2007, 76, 474–481. [Google Scholar] [CrossRef]
- Roozbeh, N.; Amirian, A.; Abdi, F.; Haghdoost, S. A systematic review on use of medicinal plants for male infertility treatment. J. Family Reprod. Health. 2021, 15, 74. [Google Scholar] [CrossRef]
- Nguyen, P.-H.; Yang, J.-L.; Uddin, M.N.; Park, S.-L.; Lim, S.-I.; Jung, D.-W.; Williams, D.R.; Oh, W.-K. Protein tyrosine phosphatase 1B (PTP1B) inhibitors from Morinda citrifolia (Noni) and their insulin mimetic activity. J. Nat. Prod. 2013, 76, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Rao, U. In Vitro Free Radical Scavenging and Reducing Potentials as well as Inhibitory Potential on α-Amylase and α-Glucosidase Activities of Fruit of Morinda citrifolia (Rubiaceae). Res. J. Pharm. Technol. 2018, 11, 4135–4142. [Google Scholar] [CrossRef]
- Khamis, M.; Talib, F.; Rosli, N.S.; Dharmaraj, S.; Mohd, K.S.; Srenivasan, S.; Latif, Z.A.; Utharkar, M.R.S. In Vitro α-amylase and α-glucosidase inhibition and increased glucose uptake of Morinda citrifolia fruit and scopoletin. Res. J. Pharm. Technol. 2015, 8, 189–193. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food. Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, J.; Li, X.; Tang, X.; Ma, S.; Lv, Y.; Yang, S. Noni (Morinda citrifolia L.) wine prevents the oxidative stress and obesity in mice induced by high-fat diet. J. Food Biochem. 2020, 44, e13460. [Google Scholar] [CrossRef]
- Nyakudya, T.; Makaula, S.; Mkumla, N.; Erlwanger, K. Dietary supplementation with coriander (Coriandrum sativum) seed: Effect on growth performance, circulating metabolic substrates, and lipid profile of the liver and visceral adipose tissue in healthy female rats. Int. J. Agric. Biol. 2014, 16, 125–131. [Google Scholar]
- Patel, D.K.; Desai, S.N.; Devkar, R.V.; Ramachandran, A. Coriandrum sativum L. aqueous extract mitigates high fat diet induced insulin resistance by controlling visceral adiposity in C57BL/6J mice. Bol. Latinoam. Caribe. Plantas. Med. Aromát. 2011, 10, 127–135. [Google Scholar]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacog. J. 2018, 10, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Dhanda, T.; Madan, V.; Beniwal, R.K. Quantitative analysis of phenols, flavonoids in different parts of Aegle marmelos (Bael) along with the evaluation of Antioxidant potential using different extracts. J. Pharmacog. Phytochem. 2020, 9, 1192–1198. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food. Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Ilavenil, S.; Karnan, M.; Yang, C.-J.; Kim, D.; Choi, K.C. Novel Bacillus ginsengihumi CMRO6 Inhibits Adipogenesis via p38MAPK/Erk44/42 and Stimulates Glucose Uptake in 3T3-L1 Pre-Adipocytes through Akt/AS160 Signaling. Int. J. Mol. Sci. 2022, 23, 4727. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.-X.; Lu, D.-Y.; Zhang, R.; Zhong, Q.-Q.; Zhao, Y.-L.; Zheng, X.-X.; Ji, S.; Wang, L.; Tang, D.-Q. Amelioration of lipid accumulations and metabolism disorders in differentiation and development of 3T3-L1 adipocytes through mulberry leaf water extract. Phytomedicine 2022, 98, 153959. [Google Scholar] [CrossRef]
- Minale, G.; Saesong, T.; Temkitthawon, P.; Waranuch, N.; Nuengchamnong, N.; Chootip, K.; Kamkaew, N.; Kongbangkerd, T.; Engsuwan, J.; Ingkaninan, K. Characterization of Metabolites in Plasma, Urine and Feces of Healthy Participants after Taking Brahmi Essence for Twelve Weeks Using LC-ESI-QTOF-MS Metabolomic Approach. Molecules 2021, 26, 2944. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, S.; Fadillah, C.; Kusuma, W. Prediction of synergistic effect between multiple compounds related to diabetes mellitus. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012038. [Google Scholar] [CrossRef]
- Alias, N.; Leow, T.; Ali, M.; Tajudin, A.; Salleh, A.; Rnzra, R. Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. Adv. Obes. Weight Manag. Control. 2017, 6, 163. [Google Scholar]
- Yu, L.; Coelho, J.E.; Zhang, X.; Fu, Y.; Tillman, A.; Karaoz, U.; Fredholm, B.B.; Weng, Z.; Chen, J.F. Uncovering multiple molecular targets for caffeine using a drug target validation strategy combining A 2A receptor knockout mice with microarray profiling. Physiol. Genom. 2009, 37, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.A.; Fernández, S.; Rodríguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, I.S.; Kim, S.H. Sinapic Acid Promotes Browning of 3T3-L1 Adipocytes via p38 MAPK/CREB Pathway. Biomed. Res. Int. 2020, 2020, 5753623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.; Mukherjee, S.; Yun, J.W. Trigonelline induces browning in 3T3-L1 white adipocytes. Phytother. Res. 2021, 35, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Concha, F.; Prado, G.; Quezada, J.; Ramirez, A.; Bravo, N.; Flores, C.; Herrera, J.J.; Lopez, N.; Uribe, D.; Duarte-Silva, L.; et al. Nutritional and non-nutritional agents that stimulate white adipose tissue browning. Rev. Endocr. Metab. Disord. 2019, 20, 161–171. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Quarta, C.; Clemmensen, C.; Zhu, Z.; Yang, B.; Joseph, S.S.; Lutter, D.; Yi, C.X.; Graf, E.; García-Cáceres, C.; Legutko, B.; et al. Molecular Integration of Incretin and Glucocorticoid Action Reverses Immunometabolic Dysfunction and Obesity. Cell Metab. 2017, 26, 620–632.e6. [Google Scholar] [CrossRef] [Green Version]
- Jayarathne, S.; Koboziev, I.; Park, O.H.; Oldewage-Theron, W.; Shen, C.L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Murumalla, R.K.; Gunasekaran, M.K.; Padhan, J.K.; Bencharif, K.; Gence, L.; Festy, F.; Césari, M.; Roche, R.; Hoareau, L. Fatty acids do not pay the toll: Effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012, 11, 175. [Google Scholar] [CrossRef] [Green Version]
- Turner, L.; Santosa, S. Putting ATM to BED: How Adipose Tissue Macrophages Are Affected by Bariatric Surgery, Exercise, and Dietary Fatty Acids. Adv. Nutr. 2021, 12, 1893–1910. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Iwasaki, Y.; Nakamura, T.; Watanabe, T.; Goto, T.; Kawada, T.; Watanabe, K.; Iwai, K. Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling. J. Nutr. Biochem. 2017, 40, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, J.Z.; Shen, J.Z.; Chen, L.; He, T.; Jin, M.W.; Liu, H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci. Rep. 2017, 7, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Zhang, Y.; Guo, J.; You, Y.; Zhan, J.; Huang, W. Chlorogenic Acid Stimulates the Thermogenesis of Brown Adipocytes by Promoting the Uptake of Glucose and the Function of Mitochondria. J. Food Sci. 2019, 84, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic acid promotes development of brown adipocyte-like phenotype in 3T3-L1 adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.H.; Yi, S.A.; Park, J.W.; Han, J.W.; Lee, J. Ginsenoside Rg3 Induces Browning of 3T3-L1 Adipocytes by Activating AMPK Signaling. Nutrients 2020, 12, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.X.; Fisher-Wellman, K.H.; Fazakerley, D.J.; Ng, Y.; Pant, H.; Li, J.; Meoli, C.C.; Coster, A.C.; Stöckli, J.; James, D.E. Selective insulin resistance in adipocytes. J. Biol. Chem. 2015, 290, 11337–11348. [Google Scholar] [CrossRef]
- Ilavenil, S.; Arasu, M.V.; Lee, J.C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, C.T.; Kim, I.H.; Kim, Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 935–939. [Google Scholar] [CrossRef]
- Sharma, T.; Kanwar, S. Phytomolecules for obesity and body weight management. J. Biochem. Cell Biol. 2018, 1, 1–8. [Google Scholar]
- Wang, H.-N.; Xiang, J.-Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Natraj, P.; Ranaweera, S.S.; Dayarathne, L.A.; Lee, Y.J.; Han, C.-H. Anti-adipogenic effect of the flavonoids through the activation of AMPK in palmitate (PA)-treated HepG2 cells. J. Vet. Sci. 2022, 23, 21256. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T.; Sato, C.; Yamamoto, R.; Watanabe, S.; Fujita, H.; Kikuno, H.; Sue, M.; Matsushima, Y. Isolation of phenolic acids and tannin acids from Mangifera indica L. kernels as inhibitors of lipid accumulation in 3T3-L1 cells. Biosci. Biotechnol. Biochem. 2022, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, J.; Li, Y.; Guo, F. Beneficial flavonoid in foods and anti-obesity effect. Food Rev. Int. 2021, 1–41. [Google Scholar] [CrossRef]
- Carvajal-Aldaz, D.; McDonough, K.; Losso, J.N. Isorhamnetin at Physiologically Attainable Nanomolar Concentrations Inhibits Adipocyte differentiation and Lipid Droplet Accumulation in vitro. J. Food Bioact. 2022, 17, 73–82. [Google Scholar] [CrossRef]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Kim, S.; Huh, S.; Kim, Y.; Kim, Y.; Byun, S.Y.; Kim, Y.-S.; Park, D. Isorhamnetin Represses Adipogenesis in 3T3-L1 Cells. Obesity 2009, 17, 226–232. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef] [Green Version]
- Sankar, M. Anti-adipogenic Activity of 3-Hydroxyflavone on 3T3-L1 Pre-adipocyte Differentiation. Asian. J. Pharm. 2022, 16, 23–29. [Google Scholar]
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Xu, C.; Li, X.; Chen, K. Hydroxylation decoration patterns of flavonoids in horticultural crops: Chemistry, bioactivity, and biosynthesis. Hort. Res. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini Rev. Org. Chem. 2022, 19, 293–318. [Google Scholar] [CrossRef]
- Inada, A.C.; Figueiredo, P.S.; dos Santos-Eichler, R.A.; Freitas, K.D.C.; Hiane, P.A.; de Castro, A.P.; Guimarães, R.D.C.A. Morinda citrifolia Linn. (Noni) and Its Potential in Obesity-Related Metabolic Dysfunction. Nutrients 2017, 9, 540. [Google Scholar] [CrossRef] [PubMed]




| Samples | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg QE/g Extract) | ||
|---|---|---|---|---|
| Aqueous Extract | Ethanolic Extract | Aqueous Extract | Ethanolic Extract | |
| A. marmelos | 108.4 ± 0.6 | 99.5 ± 0.2 | 1.02 ± 0.01 | 0.80 ± 0.02 |
| M. citrifolia | 21.8 ± 0.4 | 12.3 ± 0.6 | 1.01 ± 0.07 | 1.12 ± 0.13 |
| C. sativum | 22.3 ± 0.7 | 18.4 ± 0.4 | 0.93 ± 0.03 | 0.82 ± 0.03 |
| PTP | 62.4 ± 0.5 | 49.3 ± 0.7 | 1.19 ± 0.09 | 0.98 ± 0.07 |
| Extract/Sample | IC50 (µg/mL) | |
|---|---|---|
| Aqueous Extract | Ethanolic Extract | |
| A. marmelos | 79.1 ± 6.0 | 201.7 ± 7.8 |
| M. citrifolia | 181.7 ± 6.4 | >2000 |
| C. sativum | 139.4 ± 12.7. | >2000 |
| PTP | 92.4 ± 6.6 | 292.1 ± 32.7 |
| L-ascorbic acid | 7.2 ± 0.1 | |
| Trolox | 11.3 ± 0.7 | |
| No. | RT (min) | m/z | Adduct | MS/MS | Tentative Identification | Formula | Error(ppm) | Plant | Group of Compounds |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.855 | 104.1064 | [M]+ | 60.0798, 58.0640 | Choline | C5H14NO | 10.94 | AMC | Amino acid |
| 2 | 3.109 | 118.0853 | [M]+ | 58.0641 | Betaine | C5H12NO2 | 12.73 | A | Amino acid |
| 3 | 3.117 | 160.0957 | [M + H]+ | 88.0745, 58.0640 | Isovaleryglycine | C7H13NO3 | 6.99 | MC | Amino acid |
| 4 | 3.133 | 195.0493 | [M − H]− | 161.0250, 129.0121, 85.0239, 75.0043, 59.0096 | Gluconic acid | C6H12O7 | 8.85 | C | Monosaccharides |
| 5 | 3.187 | 116.0696 | [M + H]+ | 70.0640, 58.0640 | Proline | C5H9NO2 | 8.66 | A | Amino acid |
| 6 | 3.219 | 439.0899 | [M + CH3COO]− | 337.0640, 337.6055, 96.9641, 78.9543 | 4-Methoxybenxyl O-(2-sulfoglucoside) | C14H20O10S | 3.8 | AMC | sulfoglucoside |
| 7 | 3.338 | 144.1007 | [M + H]+ | 84.0798, 58.0641 | L-2-Amino-3-methylenehexanoic acid | C7H13NO2 | 8.36 | AMC | Amino acid |
| 8 | 3.388 | 158.1164 | [M + H]+ | 98.0939, 70.0640, 58.0640 | 3-(piperidin-3-yl)propanoic acid | C8H15NO2 | 7.31 | MC | Amino acid |
| 9 | 3.364 | 191.0572 | [M − H]− | 93.0292, 85.0246, 71.0095, 59.0101 | Quinic acid | C7H12O6 | −5.7 | A | acid |
| 10 | 3.981 | 133.0115 | [M − H]− | 114.9976, 71.0094 | Malic acid | C4H6O5 | 20.65 | AMC | acid |
| 11 | 4.477 | 189.0057 | [M − H]− | 99.0028, 83.0086, 55.0149 | Oxalosuccinic acid | C6H6O7 | −8.59 | M | acid |
| 12 | 4.721 | 290.0905 | [M-H2O-H]− | 128.0272 | Glucose-6-glutamate | C11H19NO9 | −8.14 | M | Amino sugar |
| 13 | 4.804 | 203.0208 | [M − H]− | 79.0130, 71.0089 | Oxaloglutarate | C7H8O7 | −5.29 | A | acid |
| 14 | 4.849 | 193.0328 | [M + H]+ | 111.0006, 89.0553 | Citric acid | C6H8O7 | 7.66 | AC | acid |
| 191.0209 | [M − H]− | 111.0019, 87.0029 | Citric acid | C6H8O7 | −6.11 | AC | acid | ||
| 15 | 4.849 | 175.0227 | [M + H]+ | 133.0226, 111.0007 | Dehydroascorbic acid | C6H6O6 | 5.8 | A | acid |
| 16 | 5.587 | 294.151 | [M + H]+ | 276.1413, 258.1299, 230.1364, 144.100, 86.0950 | N-(1-Deoxy-1-fructosyl)isoleucine | C12H23NO7 | 12.68 | MC | Amino sugar |
| 17 | 5.67 | 389.1138 | [M − H]− | 209.0352, 137.0526, 89.0178, 59.0092 | Menotropein | C16H22O11 | −12.5 | M | Iridoid |
| 18 | 6.056 | 117.0195 | [M − H]− | 73.0242 | Succinic acid | C4H6O4 | −1.43 | C | acid |
| 19 | 6.252 | 530.1593 | [M − H]− | 504.9040, 206.0347, 162.0470, 160.0313 | Unidentified | C | |||
| 20 | 6.565 | 389.1151 | [M − H]− | 350.6325, 330.9792, 278.4458, 210.0532, 139.0295, 100.0060, 71.0070 | Deacetyl asperulosidic acid | C16H22O11 | −12.5 | M | Iridoid |
| 21 | 7.158 | 166.0848 | [M + H]+ | 120.0791, 103.0530, 77.0376 | Phenylalanine | C9H11NO2 | 9.97 | AMC | Amino acid |
| 22 | 7.513 | 447.1354 | [M − H]− | 179.0415 | Sakuranetin | C22H24O10 | −12.81 | M | Flavonoid glycosides |
| 23 | 7.676 | 447.1346 | [M − H]− | 387.2598 | Isosakuranin | C22H24O10 | −11.02 | M | Flavonoid glycosides |
| 24 | 8.08 | 315.0552 | [M − H]− | 152.0007, 108.0132 | Isorhamnetin | C16H12O7 | −13.25 | C | Flavonoids |
| 25 | 8.605 | 188.069 | [M]+ | 146.0579, 118.0634 | N-(2,5-dihydroxyphenyl)pyridinium | C11H10NO2 | 11.45 | A | Hydroquinones |
| 26 | 8.713 | 193.0484 | [M + H]+ | 137.0568, 89.0378 | Scopoletin | C10H8O4 | 5.88 | M | coumarin |
| 27 | 8.765 | 445.1571 | [M + Cl]− | 409.1380, 361.7736, 179.0421, 59.0077 | Pteroside D | C21H30O8 | 14.31 | M | Terpene |
| 28 | 8.813 | 551.1296 | [M − H]− | - | Luteolin 7-(6″-p-benzoyglucoside) | C28H24O12 | −18.33 | M | Flavonoid glycosides |
| 29 | 8.837 | 450.1564 | [M + NH4]+ | 304.1289, 235.0563, 193.0479, 147.0416 | Asperulosidic acid | C18H24O12 | 9.33 | M | Iridoid glycoside |
| 431.1285 | [M − H]− | 251.0376, 165.0430, 89.0167, 59.0081 | Asperulosidic acid | C18H24O12 | −20.88 | M | Iridoid glycoside | ||
| 30 | 8.837 | 415.1223 | [M + H]+ | 235.0548, 147.0396 | Asperuloside | C18H22O11 | 2.86 | MC | Iridoid glycoside |
| 31 | 9.162 | 883.2506 | [M + HCOO]− | 799.2357, 750.1742, 672.1283, 568.0859, 450.6009, 343.1243, 233.1087, 176.6268, 98.5898 | Acacetin 7-(4″″-Acetylrhamnosyl)-(1-6)-glucosyl-(1-3)-(6″-acetylglucoside) | C38H46O21 | 0.86 | A | Flavonoid glycosides |
| 32 | 9.189 | 487.111 | [M − H]− | 271.0498, 153.0092 | Acacetin 7-(2″-acetylglucoside) | C24H24O11 | 27.89 | A | Flavonoid glycosides |
| 33 | 9.477 | 414.193 | [M + NH4]+ | 259.0775, 163.0569, 133.0478 | Unidentified | MC | |||
| 34 | 9.509 | 415.1452 | [M + Cl]− | 357.1600, 159.7679 | Prenyl arabinosyl-(1->6)-glucoside | C16H28O10 | −18.19 | M | Fatty acyl glycosides |
| 35 | 9.536 | 883.2502 | [M + HCOO]− | 567.0918, 387.0325, 259.0462, 165.0066 | Acacetin 7-(4″″-Acetylrhamnosyl)-(1-6)-glucosyl-(1-3)-(6″-acetylglucoside) | C38H46O21 | 1.31 | A | Flavonoid glycosides |
| 36 | 9.983 | 637.2244 | [M − H]− | 548.1696, 505.7741, 443.1224, 361.1271, 277.1135, 221.0513, 179.0444, 89.0174, 89.0171 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside | C30H38O15 | −16.64 | M | Flavonoid glycosides |
| 37 | 10.004 | 353.0935 | [M − H]− | 191.0444, 179.9262 | Caffeoyl quinic acid | C16H18O9 | −16.13 | AC | Phenolic acid |
| 38 | 637.1844 | [M − H]− | 221.0513, 179.0444, 89.0174 | Rhamnazin 3-rutinoside | C29H34O16 | −10.97 | M | Flavonoid glycosides | |
| 39 | 10.261 | 867.2521 | [M − H]− | 705.1583, 551.0980, 417.0741, 271.0386, 190.9786, 125.0144 | Sanshiside D | C39H48O22 | 5.01 | A | Iridoid glycoside |
| 40 | 10.307 | 437.1288 | [M − H]− | 386.2684, 197.2444 | Phlomiol | C17H26O13 | 2.89 | C | Iridoid glycoside |
| 41 | 385.0752 | [M − H]− | 285.7925, 191.0090, 85.0225 | Feruloylglucaric acid | C16H18O11 | 6.31 | A | Phenolic acid | |
| 42 | 10.798 | 867.2543 | [M − H]− | 705.1604, 551.0937, 389.0427, 311.0391, 125.0158 | Sanshiside D | C39H48O22 | 2.48 | A | Iridoid glycoside |
| 43 | 10.911 | 449.1176 | [M − H]− | 287.0413, 269.0311, 259.0470, 125.0154 | Eriodictyol 7-O-glucoside | C21H22O11 | −19.29 | A | Flavonoid glycosides |
| 44 | 11.394 | 325.1122 | [M + H]+ | 163.0597, 85.0275 | Moracin L | C19H16O5 | −15.84 | MC | Carbonic acids |
| 458.2196 | [M + NH4]+ | Diferuloylputrescine | C24H28N2O6 | Phenolic amide | |||||
| 45 | 11.43 | 475.1682 | [M + Cl]− | 323.0847, 263.0568, 179.0450, 115.0669, 79.0121, 59.0075 | Diferuloylputrescine | C24H28N2O6 | −8.55 | M | Phenolic amide |
| 46 | 11.693 | 611.1559 | [M + H]+ | 576.4255, 465.0904, 303.0463, 147.0633 | Rutin | C27H30O16 | 7.79 | M | Flavonoid glycosides |
| 46 | 11.828 | 609.158 | [M − H]− | 300.0108, 271.0113, 255.0165, 150.9935, 107.0051, 63.0188 | Rutin | C27H30O16 | −19.52 | M | Flavonoid glycosides |
| 47 | 11.971 | 273.0731 | [M + H]+ | Maracin J | C15H12O5 | 9.7 | A | ||
| 48 | 12.041 | 367.1097 | [M − H]− | 173.0343, 93.0267 | Feruloylquinic acid | C17H20O9 | −17.01 | A | Phenolic acid |
| 49 | 12.274 | 453.3406 | [M + H]+ | 435.3290, 376.0758, 336.2258, 285.1264, 245.1782, 210.1452, 175.0743, 139.0846, 100.0526, 55.0526 | 3-Oxo-12,18-ursadien-28-oic acid | C30H44O3 | −9.44 | MC | Triterpenoids |
| 50 | 12.394 | 433.1226 | [M − H]− | 271.0476, 150.9942, 107.0083 | 5,7,8-Trihydroxyflavanone 7-glucoside | C21H22O10 | −19.81 | A | Flavonoid glycosides |
| 51 | 12.431 | 851.2593 | [M − H]− | 689.1579, 563.1371, 401.0850, 325.5702, 255.0522, 125.0147 | Peracetylmacrophylloside D | C39H48O21 | 2.62 | A | |
| 52 | 12.772 | 648.3033 | [M + NH4]+− | 325.1082, 289.0870, 163.0578 | Nonioside B | C26H46O17 | 6.21 | M | oligosaccharides |
| 52 | 12.826 | 665.2575 | [M + Cl]− | 485.1337, 389.1604, 305.1470, 179.0456, 89.0173 | Nonioside B | C26H46O17 | −21.94 | M | oligosaccharides |
| 53 | 13.392 | 713.4895 | [M + Cl]− | 654.4719, 601.0903, 558.4126, 488.5905, 427.7981, 318.4760, 258.7616, 203.0478, 136.2926, 73.9288 | Bis{2-[2-(dodecyloxy)ethoxy]ethyl} benzene-1,2-dicarboxylate | C40H70O8 | 8.9 | MC | |
| 53 | 13.431 | 679.5083 | [M + H]+ | 552.4446, 436.3200, 336.2268, 210.1470, 100.1106 | Bis{2-[2-(dodecyloxy)ethoxy]ethyl} benzene-1,2-dicarboxylate | C40H70O8 | 8.9 | MC | |
| 54 | 14.569 | 503.2024 | [M + Cl]− | 263.0625, 143.0988, 59.0078 | Unidentified | 55 | M | ||
| 55 | 14.677 | 486.251 | [M + NH4]+ | 325.1078, 163.0576, 85.0272 | Unidentified | MC | |||
| 55 | 325.1119 | - | 85.0263 | Moracin derivative | C19H16O5 | −14.92 | MC | ||
| 56 | 14.736 | 503.2014 | - | 389.1673, 323.0829, 263.0625, 143.0989, 89.0177, 59.0082 | Unidentified | −18.14 | M | ||
| 57 | 15.267 | 289.163 | - | 127.1096, 69.0321 | Unidentified | MA | |||
| 58 | 15.274 | 503.2008 | - | 179.0436, 143.0983, 113.0153, 89.0179, 59.0073 | Unidentified | M | |||
| 59 | 15.297 | 693.2956 | [M + Cl]− | 333.1779, 221.0549, 179.0453, 119.0263, 89.0173 | Methylcellulose | C29H54O16 | 21.62 | M | |
| 60 | 17.388 | 274.2728 | [M + H]+ | 256.2618, 106.0849, 88.0747, 70.0643, 57.0689 | Hexadecasphinganine | C16H35NO2 | 4.58 | AMC | sphingoid |
| 61 | 18.065 | 763.3356 | [M − H]− | 619.1710, 403.2154, 305.1434, 277.1116, 179.0436, 115.0680 | Unidentified | M | |||
| 62 | 18.097 | 327.1683 | [M + H]+ | 259.1053, 241.0943, 198.0887, 135.0529, 106.0273, 69.0688, 51.0226 | Unidentified | A | |||
| 63 | 18.840 | 244.2613 | [M + H]+ | 226.2483, 76.0747, 58.0641 | 3-Lauryloxypropylamine | C15H33NO | 8.97 | AMC | N compound |
| 64 | 20.253 | 791.3657 | [M + Cl]− | 691.1349, 611.8238, 529.0908, 431.2437, 305.1467, 233.1283, 179.0465, 101.0156, 89.0161 | Unidentified | M | |||
| 65 | 20.68 | 286.2432 | [M − H]− | 200.0431, 146.4621, 88.0326 | Prosopinine | C16H33NO3 | −15.49 | M | Alkaloid |
| 65 | 20.708 | 288.2522 | [M + H]+ | 242.2443, 88.0745 | Prosopinine | C16H33NO3 | 3.89 | AM | Alkaloid |
| 66 | 23.223 | 293.1777 | [M − H]− | 236.0874, 221.1447, 205.1122, 148.0444, 107.0432 | Phytuberin | C17H26O4 | −6.37 | MC | Sesquiterpenoids |
| 67 | 28.584 | 307.1472 | [M + H]+ | 291.2441, 258.8319, 238.0707, 210.0759, 183.0840, 133.0842, 106.0254, 79.0407, 52.0293 | Unidentified | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngamdokmai, N.; Ingkaninan, K.; Scholfield, C.N.; Insumrong, K.; Neungchamnong, N.; Minale, G.; Warinhomhoun, S. A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits. Foods 2022, 11, 3067. https://doi.org/10.3390/foods11193067
Ngamdokmai N, Ingkaninan K, Scholfield CN, Insumrong K, Neungchamnong N, Minale G, Warinhomhoun S. A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits. Foods. 2022; 11(19):3067. https://doi.org/10.3390/foods11193067
Chicago/Turabian StyleNgamdokmai, Ngamrayu, Kornkanok Ingkaninan, C. Norman Scholfield, Kamonlak Insumrong, Nitra Neungchamnong, Genet Minale, and Sakan Warinhomhoun. 2022. "A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits" Foods 11, no. 19: 3067. https://doi.org/10.3390/foods11193067
APA StyleNgamdokmai, N., Ingkaninan, K., Scholfield, C. N., Insumrong, K., Neungchamnong, N., Minale, G., & Warinhomhoun, S. (2022). A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits. Foods, 11(19), 3067. https://doi.org/10.3390/foods11193067