Development of a Sensitive and Fast Determination Method for Trace Carbaryl Residues in Food Samples Based on Magnetic COF (TpPa-NH2)@Fe3O4 Nanoparticles and Fluorescence Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals and Reagents
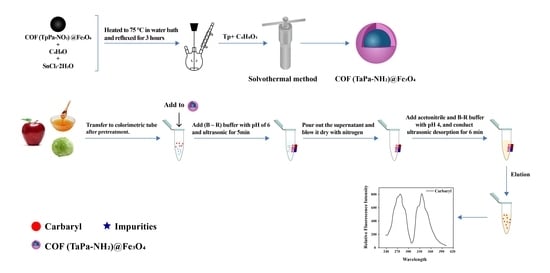
2.3. Preparation of Fe3O4 Magnetic Nanoparticles
2.4. Preparation of COF @Fe3O4 Magnetic Nanoparticles
2.5. Real Sample Preparation
2.6. MSPE Procedure
2.7. Optimization of MSPE Process
3. Results and Discussion
3.1. Optimization of the Extraction and Desorption Conditions
3.1.1. Selection of Magnetic Material
3.1.2. Effect of Sample Solution pH
3.1.3. Amount of Magnetic Adsorbent
3.1.4. Effect of the Extraction and Desorption Time
3.1.5. Optimization of Desorption Conditions
3.2. Reusability of the Magnetic Adsorbent
3.3. Excitation and Emission Spectra
3.4. Adsorption Mechanism
3.5. Analytical Performance
Analysis of Food Samples
3.6. Comparison with Other Reported Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nemati, M.; Tuzen, M.; Farazajdeh, M.A.; Kaya, S.; Afshar-Mogaddam, M.R. Development of dispersive solid-liquid extraction method based on organic polymers followed by deep eutectic solvents elution; application in extraction of some pesticides from milk samples prior to their determination by HPLC-MS/MS. Anal. Chim. Acta 2022, 1199, 339570. [Google Scholar] [CrossRef] [PubMed]
- Santalad, A.; Santalad, S.; Burakham, R.; Sakai, T.; Deming, R.L. Acid-induced cloud-point extraction coupled to spectrophotometry for the determination of carbaryl residues in waters and vegetables. Microchem. J. 2008, 90, 50–55. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, G.; Fang, G.; Wang, S. Development of a Capillary Electrophoresis-Based Immunoassay with Laser-Induced Fluorescence for the Detection of Carbaryl in Rice Samples. J. Agric. Food Chem. 2008, 56, 8832–8837. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.; Han, Y.; Shi, X. Development of a Biomimetic Enzyme-Linked Immunosorbent Assay Based on Molecularly Imprinted Polymers on Paper for the Detection of Carbaryl. Food Chem. 2018, 240, 893–897. [Google Scholar] [CrossRef]
- Zahiri, E.; Khandaghi, J.; Farajzadeh, M.A.; Afshar-Mogaddam, M.R. Combination of dispersive solid phase extraction with solidification organic drop–dispersive liquid–liquid microextraction based on deep eutectic solvent for extraction of organophosphorous pesticides from edible oil samples. J. Chromatogr. A 2020, 627, 461390. [Google Scholar] [CrossRef]
- Jia, G.; Li, L.; Qiu, J.; Wang, X.; Zhu, W.; Sun, Y.; Zhou, Z. Determination of carbaryl and its metabolite 1-naphthol in water samples by fluorescence spectrophotometer after anionic surfactant micelle-mediated extraction with sodium dodecylsulfate. Spectrochim. Acta Part A 2007, 67, 460–464. [Google Scholar] [CrossRef]
- Faraji, M.; Noormohammadi, F.; Moradi, M. Supramolecular-based solvent microextraction of carbaryl in water samples followed by high performance liquid chromatography determination. Int. J. Environ. Anal. Chem. 2017, 97, 730–742. [Google Scholar] [CrossRef]
- Li, W.K.; Feng, J.T.; Ma, Z.Q. Nitrogen, sulfur, boron and flavonoid moiety co- incorporated carbon dots for sensitive fluorescent detection of pesticides. Carbon 2020, 161, 685–693. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z.G. Creation of carbazole-based fluorescent porous polymers for recognition and detection of various pesticides in water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef]
- Totti, S.; Fernandez, M.; Fernandez, S.; Fini, F.; Girotti, S. Application of matrix solid phase dispersion to the determination of imidacloprid carbaryl aldicarb and their main metabolites in honeybees by liquid chromatography-mass spectrometry detection. Talanta 2006, 69, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Girotti, S.M.; Alves, R.D.; Queiroz, T.C. Optimization and validation of liquid-liquid extraction with low temperature partitioning for determination of carbamates in water. Anal. Chim. Acta. 2010, 671, 41–47. [Google Scholar]
- Fu, R. Analysis of Carbaryl and Carbofuran in Drinking Water with Post-Column Derivatization Using Agilent’s New LC Column and SampliQ SPE Cartridges. Agil. Technol. 2008, 5989–9802. [Google Scholar]
- Talebianpoor, M.S.; Khodadoust, S.; Mousavi, A.; Mahmoudi, R.; Mohammadi, J. Preconcentration of carbamate insecticides in water samples by using modifified stir bar with ZnS nanoparticles loaded on activated carbon and their HPLC determination: Response surface methodology. Microchem. J. 2017, 130, 64–70. [Google Scholar] [CrossRef]
- Sun, X.Z.; Fu, Z.B.; Jiang, T.Y.; Ning, F.H.; Cheng, Y.Z.; Fu, T.H. Application of β-Cyclodextrin metal-organic framework/titanium dioxide hybrid nanocomposite as dispersive solid-phase extraction adsorbent to organochlorine pesticide residues in honey samples. J. Chromatogr. A 2022, 1633, 462750. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liu, X.L.; Wang, J.T.; Wang, C.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon as an adsorbent for the magnetic solid-phase extraction of chlorophenols from mushroom sample. Chin. Chem. Lett. 2017, 27, 783–788. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, M.; Duan, W.; Wang, X.; Zhao, H.; Guo, L. Phytic acid-stabilized super-amphiphilic Fe3O4-graphene oxide for extraction of polycyclic aromatic hydrocarbons from vegetable oils. Food Chem. 2017, 235, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cheshari, E.C.; Ren, X.; Li, X. Core-shell magnetic Ag-molecularly imprintedcomposite for surface enhanced Raman scattering detection of carbaryl. J. Environ. Sci. Health Part B 2021, 56, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Song, Y.; Liu, W.; Wang, Z.; Wang, C. Combination of magnetic solid-phase extraction and HPLC-UV for simultaneous determination of four phthalate esters in plastic bottled juice. Food Chem. 2021, 339, 127855. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.; Xing, F.; Liu, W.; Wang, L.C.; Wang, Z. Graphene intercalated with carbon nanosphere: A novel solid-phase extraction sorbent for five carbamate pesticides. Microchim. Acta 2020, 187, 521. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Fu, Z.; Li, Y.; Su, X.; Zou, L.; Yang, Y. Preparation and characterization of magnetic molecular imprinted polymers with ionic liquid for the extraction of carbaryl in food. Anal. Bioanal.Chem. 2020, 412, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; G’andara, F.; Yaghi, O.M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.X.; Yan, X.P. Controllable preparation of core-shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. 2017, 53, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, L.; Zhang, R.; Zhang, Y.; Chen, J.; Zhang, W. Solid-phase extraction based on magnetic core-shell silica nanoparticles coupled with gas chromatography-mass spectrometry for the determination of low concentration pesticides in aqueous samples. J. Sep. Sci. 2012, 25, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wu, H.; Yang, L.; Wang, Z.; Wang, J. Use of a magnetic covalent organic framework material with a large specific surface area as an effective adsorbent for the extraction and determination of six fluoroquinolone antibiotics by HPLC in milk sample. J. Sep. Sci. 2020, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Lv, S.W.; Yuan, X.Y.; Wang, S. Facile construction of magnetic core-shell covalent organic frameworks as efficient solid-phase extraction adsorbents for highly sensitive determination of sulfonamide residues against complex food sample matrices. RSC Adv. 2019, 9, 14247–14253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, W.; Wang, Q.; Wu, Q.; Wang, Z. Constructing magnetic covalent organic framework EB-COF@Fe3O4 for sensitive determination of five benzoylurea insecticides. Food Chem. 2022, 382, 132362. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Research Service (ARS) Solubility of Carbaryl. Available online: https://www.ars.usda.gov/Services/docs.htm?docid=14199 (accessed on 25 August 2022).
- Gao, N.; Wu, H.; Chang, Y.; Guo, X.; Zhang, L.; Du, L.; Fu, Y. Mixed micelle cloud point-magnetic dispersive l-solid phase extraction of doxazosin and alfuzosin. Spectrochim. Acta Part A 2015, 134, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guan, P.; Yang, L.; Li, R. Separation and Enrichment of Five Fluoroquinolones in Milk by Nitro Modified Magnetic Covalent Organic Framework Polymer. Chin. J. Anal. Lab. 2021, 40, 2096–4188. [Google Scholar]
- Wang, Y.; Wu, S.; Wu, D.; Shen, J.; Wei, Y.; Wang, C. Amino bearing core-shell structured magnetic covalent organic framework nanospheres: Preparation, postsynthetic modifification with phenylboronic acid and enrichment of monoamine neurotransmitters in human urin. Anal. Chim. Acta 2020, 1093, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Gao, J.; Wu, Q.; Wu, C.; Wang, Z. A Graphene Oxide-Based Composite for Solid-Phase Extraction of Carbamate Pesticides from Vegetables. Food Anal. Methods 2020, 13, 690–698. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Zhang, Y.; Chen, L.; Dang, X.; Ai, Y.; Chen, H. Fe3O4 nanoparticles coated with polyhedral oligomeric silsesquioxanes and β-cyclodextrin for magnetic solid phase extraction of carbaryl and carbofuran. J. Sep. Sci. 2020, 43, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, N.; Cui, L.; Wang, X.; Zhao, S. Recent application of magnetic solid phase extraction for food safety analysis. TrAC Trends Anal. Chem. 2019, 120, 115632. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Z.; Nie, Z.; Deng, H.; Hu, H.; Yao, S. Resurfaced fluorescent protein as a sensing platform for label-free detection of copper (II) ion and acetylcholinesterase activity. Anal. Chem. 2015, 87, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Khosrowshahi, E.M.; Ghalkhani, M.; Afshar-Mogaddam, M.R.; Farajzadeh, M.A.; Sohouli, E.; Nemati, M. Evaluation of MXene as an adsorbent in dispersive solid phase extraction of several pesticides from fresh fruit juices prior to their determination by HPLC-MS/MS. Food Chem. 2022, 386, 13277. [Google Scholar] [CrossRef]




| Analytes | Structure | Molecular Weight | H Bond Acceptors | H Bond Donors | LogKow a | ER b (%) |
|---|---|---|---|---|---|---|
| Carbaryl |  | 200.21 | 2 | 1 | 1.85 | 99.5 |
| Metoxuron |  | 228.68 | 4 | 1 | 1.64 | 55.2 |
| Isoproturon |  | 206.28 | 3 | 1 | 2.87 | 76.1 |
| Carbofuran |  | 221.25 | 4 | 1 | 1.52 | 47.6 |
| Propoxur |  | 209.2 | 4 | 1 | 1.90 | 67.3 |
| Analytes | Linear Equation (µg·kg−1) | Linear Range (µg·kg−1) | R2 | LOD (µg·kg−1) | RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day Precision at the Concentrations of (µg·kg−1) | Inter-Day Precision at the Concentrations of (µg·kg−1) | |||||||||
| 2 | 50 | 100 | 2 | 50 | 100 | |||||
| Carbaryl | y = 7.42c + 36.2 | 0.2–120 | 0.9997 | 0.012 | 1.6 | 2.2 | 2.5 | 2.0 | 2.6 | 3.3 |
| Sample | Original (µg·kg−1) | Added (µg·kg−1) | Found (µg·kg−1) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|---|
| Honey | ND | 1 | 1.02 | 102.3 | 2.5 |
| 15 | 15.76 | 105.1 | 1.9 | ||
| 30 | 29.86 | 99.5 | 3.2 | ||
| Cabbage | ND | 1 | 1.07 | 107.4 | 1.6 |
| 15 | 15.72 | 104.8 | 3.1 | ||
| 30 | 30.22 | 100.7 | 1.8 | ||
| Apple | ND | 1 | 0.96 | 96.0 | 3.6 |
| 15 | 15.12 | 100.8 | 2.2 | ||
| 30 | 29.89 | 99.6 | 2.6 |
| Extraction Method | Technique | Analytes | Sample | Extraction Time (min) | Linear Range (µg·kg−1) | LOD (µg·kg−1) | RSD (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| CPE | Visible Spectrophotometry | Carbaryl | Six vegetables | 4 | 100–7000 | 50 | 2.3 | [3] |
| DLLME | HPLC | Carbaryl | Cucumber Spinach | 250 | 1–500 | 0.3–1 | - | [8] |
| LLE | LC-MS | Carbaryl | honeybees | 13 | 4–9 | 3 | ≤14 | [11] |
| LLE | HPLC-UV | Carbaryl | Drinking Water | 10 | 0.005–0.01 | 0.001 | 4.6 | [12] |
| SPE | HPLC | Carbaryl | Water | - | 0.01 | 0.01 | 1.8 | [13] |
| SBSE | HPLC | Carbaryl | Water Samples | 23 | 0.002–30 | 0.0003 | 3.3–4.5 | [14] |
| MSPE | Fluorimetry | Carbaryl | Honey cabbage apple | 5 a | 0.2–120 | 0.012 | 1.6–3.6 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wu, H.; Jing, X.; Yu, Y.; Yan, Z.; Zhang, J. Development of a Sensitive and Fast Determination Method for Trace Carbaryl Residues in Food Samples Based on Magnetic COF (TpPa-NH2)@Fe3O4 Nanoparticles and Fluorescence Detection. Foods 2022, 11, 3130. https://doi.org/10.3390/foods11193130
Du J, Wu H, Jing X, Yu Y, Yan Z, Zhang J. Development of a Sensitive and Fast Determination Method for Trace Carbaryl Residues in Food Samples Based on Magnetic COF (TpPa-NH2)@Fe3O4 Nanoparticles and Fluorescence Detection. Foods. 2022; 11(19):3130. https://doi.org/10.3390/foods11193130
Chicago/Turabian StyleDu, Juanli, Hao Wu, Xu Jing, Yonghe Yu, Zhisheng Yan, and Jianhai Zhang. 2022. "Development of a Sensitive and Fast Determination Method for Trace Carbaryl Residues in Food Samples Based on Magnetic COF (TpPa-NH2)@Fe3O4 Nanoparticles and Fluorescence Detection" Foods 11, no. 19: 3130. https://doi.org/10.3390/foods11193130
APA StyleDu, J., Wu, H., Jing, X., Yu, Y., Yan, Z., & Zhang, J. (2022). Development of a Sensitive and Fast Determination Method for Trace Carbaryl Residues in Food Samples Based on Magnetic COF (TpPa-NH2)@Fe3O4 Nanoparticles and Fluorescence Detection. Foods, 11(19), 3130. https://doi.org/10.3390/foods11193130