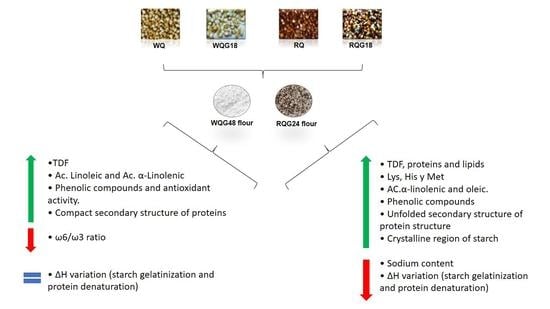
Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Water Activity of Quinoa Flours
2.3. Chemical Analysis of Quinoa Flours
2.3.1. Proximal Composition
2.3.2. Non-Fiber Carbohydrates
2.4. Total Phenols Content and Antioxidant Activity
2.4.1. Extraction Process
2.4.2. Total Polyphenols
2.4.3. Antioxidant Activity
Ferric ion Reducing Antioxidant Power Assay (FRAP)
Free Radical Scavenger Activity on 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
2.5. Mineral Composition
2.6. Fatty Acid Profile
2.7. Amino Acid Composition
2.8. Structural and Thermal Characterization of Protein and Starch
2.8.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8.3. Differential Scanning Calorimetry (DSC)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Water Availability and Chemical Composition of Quinoa Flours
3.2. Changes in Total Phenolic Compounds and Antioxidant Activity
3.3. Minerals
3.4. Fatty Acids
3.5. Amino Acids Composition
3.6. Protein Fractions
3.7. Secondary Structure of Proteins and Starch Organization
3.8. Thermal Properties
3.9. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Kozioł, M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Schlick, G.; Bubenheim, D.L. Quinoa: Candidate crop for NASA’s controlled ecological life support systems. In Progress in New Crops; Ashs Press: Alexandria, VA, USA, 1996; pp. 632–640. [Google Scholar]
- FAO. 2013 International Year of Quinoa Secretariat; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Available online: https://www.fao.org/quinoa-2013/es/?no_mobile=1 (accessed on 15 August 2022).
- Nelson, K.; Stojanovska, L.; Vasiljevic, T.; Mathai, M. Germinated grains: A superior whole grain functional food? Can. J. Physiol. Pharmacol. 2013, 91, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Fouad, A.A.; Rehab, F.M. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhalifa, A.E.O.; Bernhardt, R.; Cardone, G.; Marti, A.; Iametti, S.; Marengo, M. Physicochemical properties of sorghum flour are selectively modified by combined germination-fermentation. J. Food Sci. Technol. 2017, 54, 3307–3313. [Google Scholar] [CrossRef]
- Cardone, G.; D’Incecco, P.; Pagani, M.A.; Marti, A. Sprouting improves the bread-making performance of whole wheat flour (Triticum aestivum L.). J. Sci. Food Agric. 2020, 100, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef]
- Padmashree, A.; Negi, N.; Handu, S.; Khan, M.A.; Semwal, A.D.; Sharma, G.K. Effect of germination on nutritional, antinutritional and rheological characteristics of quinoa (Chenopodium quinoa). Def. Life Sci. J. 2018, 4, 55–60. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Leguía-Damiano, S.; Calla-Florez, M.; Zamalloa-Puma, L.M.; Colque-Condeña, L. Phenolic compounds, antioxidant capacity, and protein content of three varieties of germinated quinoa (Chenopodium quinoa willd). Ing. Investig. 2021, 41, e89831. [Google Scholar] [CrossRef]
- Guardianelli, L.M.; Salinas, M.V.; Puppo, M.C. Chemical and thermal properties of flours from germinated amaranth seeds. J. Food Meas. Charact. 2019, 13, 1078–1088. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemists (AACC International), 10th ed.; The American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- Eliasson, A.C.; Gudmundsson, M. 10 Starch. In Carbohydrates in Food; Eliasson, A.C., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 159, p. 391. [Google Scholar]
- Salinas, M.V.; Guardianelli, L.M.; Sciammaro, L.P.; Picariello, G.; Mamone, G.; Puppo, M.C. Nutritional ingredient by-product of the pistachio oil industry: Physicochemical characterization. J. Food Sci. Technol. 2021, 58, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous white–blue LED light exposition delays postharvest senescence of broccoli. LWT-Food Sci. Technol. 2016, 65, 495–502. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Ancona, D.B.; Guerrero, L.C.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Yust, M.A.M.; Pedroche, J.; Girón-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Gerbino, E.; Mobili, P.; Tymczyszyn, E.; Fausto, R.; Gómez-Zavaglia, A. FTIR spectroscopy structural analysis of the interaction between Lactobacillus kefir S-layers and metal ions. J. Mol. Struct. 2011, 987, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef]
- Menegassi, B.; Pilosof, A.M.; Arêas, J.A. Comparison of properties of native and extruded amaranth (Amaranthus cruentus L.–BRS Alegria) flour. LWT-Food Sci. Technol. 2011, 44, 1915–1921. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2012. Grupo InfoStat, FCA. 2012. Available online: www.infostat (accessed on 1 May 2021).
- Fontana, A.J. Understanding the importance of water activity in food. Cereal Foods World 2000, 45, 7–10. [Google Scholar]
- Antezana, R.N.V.; Ticona, G.M.; Yucra, F.E.Z.; Cayllahua, D.Q.; Alejo, R.M.; Churqui, U.J.M. Efecto de la germinación y cocción en las propiedades nutricionales de tres variedades de quinua (Chenopodium quinoa Willd.). Rev. Investig. Altoandinas 2015, 17, 169–172. [Google Scholar] [CrossRef]
- Demir, B.; Bilgiçli, N. Changes in chemical and anti-nutritional properties of pasta enriched with raw and germinated quinoa (Chenopodium quinoa Willd.) flours. J. Food Sci. Technol. 2020, 57, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.; Al-Jumayi, H.A.; Elhendy, H.A. Effect of germination on the nutritional profile of quinoa (Cheopodium quinoa Willd.) seeds and its anti-anemic potential in Sprague–Dawley male albino rats. Cereal Chem. 2021, 98, 315–327. [Google Scholar] [CrossRef]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Effect of germination on proximate principles, minerals and anti-nutrients of flaxseeds. Asian J. Dairy Food Res. 2017, 36, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.L.C.; Cox, M. Lehninger Principles of Biochemistry, 4th ed.; WH Freeman: New York, NY, USA, 2005; pp. 601–652. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Park, S.H.; Morita, N. Changes of bound lipids and composition of fatty acids in germination of quinoa seeds. Food Sci. Technol. Res. 2007, 10, 303–306. [Google Scholar] [CrossRef] [Green Version]
- FAO; OMS; UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/OMS/UNU Expert Consultation; Organización mundial de la salud (OMS): Geneva, Switzerland, 2007. [Google Scholar]
- Bhathal, S.K.; Kaur, N.; Gill, J. Effect of processing on the nutritional composition of quinoa (Chenopodium quinoa Willd). Agric. Res. J. 2017, 54, 90–93. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.; Deleu, L.J.; Delcour, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocoll. 2021, 115, 106604. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Lobo, M.; Sammán, N. 12th IFDC 2017 Special Issue–Influence of germination of quinoa (Chenopodium quinoa) and amaranth (Amaranthus) grains on nutritional and techno-functional properties of their flours. J. Food Compos. Anal. 2019, 84, 103290. [Google Scholar] [CrossRef]




| Flours | Fructose (%) | Glucose (%) | Sucrose (%) | Starch (%) |
|---|---|---|---|---|
| WQ | 0.22 ± 0.04 a | 0.91 ± 0.01 a | 0.80 ± 0.10 a | 57.72 ± 0.78 a |
| WQG18 | 0.34 ± 0.03 b | 1.49 ± 0.01 b | 0.92 ± 0.06 ab | 57.72 ± 0.82 a |
| WQG24 | 0.44 ± 0.02 c | 1.53 ± 0.02 bc | 0.96 ± 0.12 ab | 59.63 ± 0.53 b |
| WQG48 | 0.53 ± 0.02 d | 1.58 ± 0.01 c | 1.00 ± 0.08 b | 57.06 ± 0.84 a |
| RQ | 0.18 ± 0.01 a | 0.80 ± 0.00 a | 0.61 ± 0.10 a | 53.11 ± 1.71 b |
| RQG18 | 0.30 ± 0.01 b | 1.46 ± 0.01 b | 0.89 ± 0.10 b | 56.2 ± 1.5 c |
| RQG24 | 0.37 ± 0.01 c | 1.51 ± 0.07 b | 0.98 ± 0.05 b | 54.93 ± 0.91 b |
| RQG48 | 0.48 ± 0.01 d | 1.52 ± 0.01 b | 0.96 ± 0.07 b | 48.97 ± 0.98 a |
| Total Phenolics Content—Antioxidant Activity | Minerals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Flours | Folin (mg GAE/100 g Flour) | DPPH (EC50) (mg/µL) | FRAP (mg Trolox/100 g Flour) | Sodium (ppm) | Iron (ppm) | Zinc (ppm) | Calcium (ppm) | ||
| WQ | 94.3 ± 12.4 a | 22.1 ± 0.2 b | 113.7 ± 0.8 a | 200 ± 4 a | 47.3 ± 1.3 b | 27.2 ± 0.1 b | 438 ± 25 a | ||
| WQG18 | 111.1±3.8 b | 20.8 ± 0.7 a | 123.4 ± 0.7 c | 345 ± 10 b | 51.6 ± 1.1 c | 56.1 ± 0.4 c | 738 ± 57 b | ||
| WQG24 | 105.6±8.5 b | 20.6 ± 0.0 a | 120.1 ± 0.5 b | 352 ± 17 b | 45.5 ± 0.9 b | 26.3 ± 0.5 b | 766 ± 53 b | ||
| WQG48 | 121.5 ± 0.7 c | 19.9 ± 0.2 a | 127.4 ± 0.7 d | 367 ± 18 b | 39.7 ± 0.7 a | 24.3 ± 0.2 a | 729 ± 50 b | ||
| RQ | 113.7 ± 0.8 a | 7.6 ± 0.0 a | 72.0 ± 1.4 c | 914 ± 66 c | 33.6 ± 0.8 b | 21.1 ± 0.1 a | 413 ± 16 a | ||
| RQG18 | 123.4± 0.7 c | 8.2 ± 0.0 b | 62.6 ± 1.7 a | 684± 33 b | 35.8 ± 0.6 c | 36.8 ± 0.5 b | 988 ± 26 b | ||
| RQG24 | 120.1±0.5 b | 8.0 ± 0.1 b | 67.2 ± 0.9 b | 503 ± 24 a | 31.9 ± 0.4 a | 20.9 ± 0.4 a | 427 ± 10 a | ||
| RQG48 | 127.4±0.7 d | 8.7 ± 0.2 c | 63.0 ± 1.9 a | 682 ± 52 b | 35.5 ± 0.8 c | 21.0 ± 0.4 a | 951 ± 82 b | ||
| Unsaturated fatty acids (g/100 g lipids) | |||||||||
| Flours | Oleic acid (18:1-ω9) | Linoleic acid (18:2-ω6) | α-Linolenic acid (18:3-ω3) | Eicosenoic acid (20:1-ω9) | ω6/ω3 ratio | ||||
| WQ | 30.0 ± 0.0 c | 49.0 ± 0.1 a | 8.7 ± 0.0 a | 1.3 ± 0.0 a | 5.6 ± 0.0 b | ||||
| WQG18 | 30.0 ± 0.1 c | 49.0 ± 0.0 a | 8.7 ± 0.1 a | 1.4 ± 0.1 a | 5.6 ± 0.1 b | ||||
| WQG24 | 29.8 ± 0.0 b | 48.9 ± 0.0 a | 8.9 ± 0.0 b | 1.4 ± 0.0 a | 5.5 ± 0.0 b | ||||
| WQG48 | 28.4 ± 0.1 a | 49.8 ± 0.2 b | 9.4 ± 0.0 c | 1.3 ± 0.0 a | 5.3 ± 0.0 a | ||||
| RQ | 27.0 ± 0.0 a | 53.2 ± 0.1 c | 6.2 ± 0.0 a | 1.3 ± 0.1 a | 8.6 ± 0.1 d | ||||
| RQG18 | 28.0 ± 0.1 c | 52.2 ± 0.0 a | 6.6 ± 0.0 b | 1.3 ± 0.1 a | 7.9 ± 0.0 c | ||||
| RQG24 | 27.8 ±0.0 bc | 52.1 ± 0.0 a | 6.8 ± 0.0 d | 1.4 ± 0.0 a | 7.6 ± 0.0 a | ||||
| RQG48 | 27.6 ± 0.2 b | 52.3 ± 0.1 b | 6.7 ± 0.0 c | 1.4 ± 0.0 a | 7.8 ± 0.0 b | ||||
| Essential amino acids (mg/100 g de proteins) | |||||||||
| Flours | Lysine (4.5 *) | Histidine (1.5 *) | Valine (3.9 *) | Isoleucine (3.0 *) | Leucine (5.9 *) | Methionine (1.6 *) | Tyrosine + Phenylalanine (3.8 *) | Threonine (2.3 *) | Tryptophan (0.6 *) |
| WQ | 16.6 ± 1.6 b | 6.4 ± 0.8 a | 6.5 ± 0.3 bc | 15.8 ± 0.2 b | 10.8 ± 0.8 a | 1.2 ± 0.6 ab | 10.9 ± 1.8 b | 8.1 ± 0.5 b | 1.5 ± 0.1 a |
| WQG18 | 15.6 ± 1.0 b | 5.4 ± 0.8 a | 6.7 ± 0.5 c | 15.4 ± 0.8 b | 10.8 ± 0.9 a | 1.7 ± 0.3 b | 10.8 ± 0.8 b | 7.7 ± 0.4 b | 1.3 ± 0.0 a |
| WQG24 | 11.9 ± 2.5 a | 12.0 ± 0.9 c | 4.4 ± 0.1 a | 12.2 ± 2.2 a | 10.8 ± 0.1 a | 0.9 ± 0.1 a | 8.6 ± 1.2 a | 6.1 ± 0.2 a | 1.5 ± 0.3 a |
| WQG48 | 12.5 ± 0.9 a | 10.5 ± 1.9 b | 5.7 ± 0.4 b | 12.2 ± 0.5 a | 9.5 ± 0.1 a | 0.8 ± 0.1 a | 8.5 ± 0.5 a | 6.5 ± 0.3 a | 1.6 ± 0.2 a |
| RQ | 13.2 ± 0.6 a | 6.8 ± 1.5 a | 5.5 ± 0.3 a | 14.0 ± 1.2 a | 9.5 ± 0.3 ab | 1.1 ± 0.2 b | 9.4 ± 0.6 a | 6.7 ± 0.7 ab | 1.2 ± 0.1 a |
| RQG18 | 13.3 ± 0.7 a | 7.0 ± 1.1 a | 5.7 ± 0.1 a | 13.9 ± 0.7 a | 10.5 ± 0.4 b | 1.3 ± 0.1 b | 9.3 ± 0.9 a | 7.4 ± 0.4 b | 1.4 ± 0.1 a |
| RQG24 | 13.9 ± 2.1 a | 22.1 ±1.7 b | 5.1 ± 0.6 a | 12.1 ± 1.1 a | 9.3 ± 0.7 a | 2.5 ± 0.4 c | 9.1 ± 0.5 a | 6.5 ± 0.1 a | 1.7 ± 0.4 a |
| RQG48 | 12.2 ± 0.2 a | 7.0 ± 1.4 a | 5.7 ± 0.5 a | 12.7 ± 0.1 a | 9.1 ± 0.3 a | 0.3 ± 0.1 a | 8.5 ± 0.9 a | 6.9 ± 0.2 ab | 1.3 ± 0.1 a |
| Secondary Structure | Wavenumber (cm−1) | Flours | |||
|---|---|---|---|---|---|
| WQ | WQG48 | RQ | RQG24 | ||
| β-sheet parallel intermolecular | 1620 | 42 ± 2.5 b | 37.3 ± 2.4 ab | 30.3 ± 4.1 a | 31.1 ± 3.0 a |
| β-sheet parallel intramolecular | 1636 | 18.5 ± 1.8 a | 19.7 ± 1.9 ab | 20.8 ± 1.2 a | 26.9 ± 2.8 b |
| Random coil | 1650 | 21.6 ± 0.5 b | 17.9 ± 2.1 ab | 27.1 ± 0.7 b | 25.2 ± 3.0 ab |
| α-helix | 1661 | 6.7 ± 1.0 a | 11.3 ± 0.2 c | 10.7 ± 0.4 b | 8.8 ± 1.5 a |
| β-turn | 1674 | 6.4 ± 1.1 a | 6.1 ± 1.4 a | 5.7 ± 0.05 a | 7.0 ± 0.1 b |
| β-sheet antiparallel intramolecular | 1684 | 6.0 ± 0.8 a | 7.5 ± 0.2 ab | 6.6 ± 0.7 b | 5.1 ± 0.1 a |
| Starch | Wavenumber (cm−1) | ||||
| Area ratio (crystalline) | 1045/1022 | 0.36 ± 0.02 ab | 0.37 ± 0.01 ab | 0.35 ± 0.05 a | 0.45 ± 0.03 b |
| Area ratio (amorphous) | 1022/995 | 0.51 ± 0.04 ab | 0.48 ± 0.01 a | 0.56 ± 0.05 b | 0.40 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardianelli, L.M.; Salinas, M.V.; Brites, C.; Puppo, M.C. Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours. Foods 2022, 11, 3272. https://doi.org/10.3390/foods11203272
Guardianelli LM, Salinas MV, Brites C, Puppo MC. Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours. Foods. 2022; 11(20):3272. https://doi.org/10.3390/foods11203272
Chicago/Turabian StyleGuardianelli, Luciano Martín, María Victoria Salinas, Carla Brites, and María Cecilia Puppo. 2022. "Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours" Foods 11, no. 20: 3272. https://doi.org/10.3390/foods11203272
APA StyleGuardianelli, L. M., Salinas, M. V., Brites, C., & Puppo, M. C. (2022). Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours. Foods, 11(20), 3272. https://doi.org/10.3390/foods11203272