Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities
Abstract
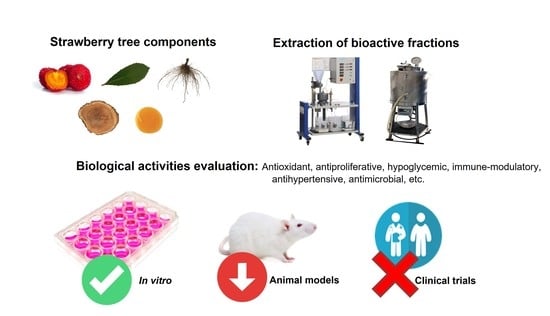
:1. Introduction
2. Antioxidant Activity
3. Antiproliferative/Antitumoral Activity
4. Hypoglycemic Activity
5. Anti-Inflammatory/Immune-Modulatory Activity
6. Antihypertensive/Vasodilatory Activity
7. Antimicrobial/Antiviral Activity
8. Other Biological Activities
9. Conclusions, Current Limitations and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus species (Ericaceae) as source of valuable bioactive products. Crit. Rev. Food Sci. Nutr. 2019, 59, 864–881. [Google Scholar] [CrossRef]
- Gul Sarikaya, A.; Orucu, O.K. Maxent modeling for predicting the potential distribution of Arbutus andrachne L. belonging to climate change in Turkey. Kuwait J. Sci. 2021, 48, 1–13. [Google Scholar] [CrossRef]
- Serçe, S.; Özgen, M.; Torun, A.A.; Ercisli, S. Chemical composition, antioxidant activities and total phenolic content of Arbutus andrachne L. (Fam. Ericaceae) (the Greek strawberry tree) fruits from Turkey. J. Food Compos. Anal. 2010, 23, 619–623. [Google Scholar] [CrossRef]
- Salas-Pascual, M.; Déniz Suárez, E.A. News and precisions about the distribution of the species of the genus Arbutus L. (Ericaceae) in Gran Canaria (Canary Islands). Bot. Complut. 2019, 43, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Kabiel, H.F.; Hegazy, A.K.; Lovett-Doust, L.; Al-Rowaily, S.L.; Borki, A.E.N. Demography of the threatened endemic shrub, Arbutus pavarii, in the Al-Akhdar mountainous landscape of Lybia. J. For. Res. 2016, 27, 1295–1303. [Google Scholar] [CrossRef]
- Silva-González, E.; Aguirre-Calderón, O.A.; Rodríguez, E.A.; González Tagle, M.A.; Treviño-Garza, E.J.; Corral-Rivas, J.J. Assessment of the forest harvesting effect on the diversity and structure of a temperate forest in the state of Durango. Rev. Mex. Cienc. For. 2022, 13, 103–132. [Google Scholar] [CrossRef]
- Kamakura, R.P.; DeWald, L.E.; Sniezko, R.A.; Elliot, M.; Chastagner, G.A. Using differences in abiotic factors between seed origin and common garden sites to predict performance of Pacific madrone (Arbutus menziesii Pursh). For. Ecol. Manag. 2021, 497, 119487. [Google Scholar] [CrossRef]
- Sorensen, P.D. Arbutus tessellata (Ericaceae), new from Mexico. Brittonia 1987, 39, 263–267. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Santiago-García, W.; Suárez-Mota, M.E.; Esquivel-Reyes, H.H.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G. Development and validation of an analytical method for condensed tannin extracts obtained from the bark of four tree species using HPLC. Wood Res. 2021, 66, 171–182. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Daskalakis, I.; Biniari, K.; Papafotiou, M. Comaprative study of polyphenolic content and antioxidant capacity in fruits of Arbutus unedo, A. andrachne and their natural hybrid A. × andrachnoides. Notulae Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12165. [Google Scholar] [CrossRef]
- Ait Lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan strawberry tree (Arbutus unedo L.) fruits: Nutritional value and mineral composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Arbutus unedo L.: From tradicional medicine to potential uses in modern pharmacotherapy. J. Ethnoparmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Benchikh, Y.; Sahli, S.; Alleg, M.; Mohellebi, N.; Gagaoua, M. Optimised statistical extraction of anthocyanins from Arubtus unedo L. fruits and preliminary supplementation assays in yoghurt. Int. J. Dairy Technol. 2021, 74, 344–351. [Google Scholar] [CrossRef]
- Pinheiro, J.; Rodrigues, S.; Mendes, S.; Maranhao, P.; Ganhao, R. Impacto f aqueous extract of Arbutus unedo fruits on limpets (Patella spp.) pâté during storage: Proximate composition, physicochemical quality, oxidative stability, and microbial development. Foods 2020, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Et-Touys, A.; Dakka, N.; Fellah, H.; Abrini, J.; Bakri, Y. Antileishmanial potential of medicinal plant extracts from the North-West of Morocco. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 50–54. [Google Scholar] [CrossRef]
- Twilley, D.; Langhansova, L.; Palaniswamy, D.; Lall, N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S. Afr. J. Bot. 2017, 112, 494–500. [Google Scholar] [CrossRef]
- Selen Isbilir, S.; Yagar, H. Determination of antioxidant activities of strawberry tree (Arbutus unedo L.) flowers and fruits at different ripening stages. Acta Sci. Pol. 2012, 11, 223–237. [Google Scholar]
- Afrin, S.; Forbes-Hernández, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A comparison with manuka honey. Int. J. Molec. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [Green Version]
- Graikou, K.; Andreou, A.; Chinou, I. Chemical profile of Greek Arbutus unedo honey: Biological properties. J. Apic. Res. 2022, 61, 100–106. [Google Scholar] [CrossRef]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.A. Authentication of strawberry tree (Arbutus unedo L.) honeys from southern Europe based on compositional parameters and biological activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crop. Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Dib, M.E.A.; Allali, H.; Bendiabdellah, A.; Meliani, N.; Tabti, B. Antimicrobial activity and phytochemical screening of Arbutus unedo L. J. Saudi Chem. Soc. 2013, 17, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Mrabti, H.N.; el Abbes Faouzi, M.; Mayuk, F.M.; Makrane, H.; Limas-Nzouzi, N.; Dibong, S.D.; Cherrah, Y.; Elombo, F.K.; Gressier, B.; Desjeux, J.F.; et al. Arbutus unedo L. (Ericaceae) inhibits intestinal glucose absorption and improves glucose tolerance in rodents. J. Ethnoparmacol. 2019, 235, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Bouyahya, A.; Ed-Dra, A.; Kachmar, M.R.; Mrabti, N.N.; Benali, T.; Shariati, M.A.; Ouahbi, A.; Doudach, L.; Faouzi, M.E.A. Polyphenolic profile and biological properties of Arbutus unedo root extracts. Eur. J. Integr. Med. 2021, 101266. [Google Scholar] [CrossRef]
- Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Tahri, A.; Legssyer, A.; Hoerter, J.; Fischmeister, R. Arbutus unedo induces endothelium-dependent relaxation of the isolated rat aorta. Phytother. Res. 2002, 16, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Prieto, M.A.; Vazquez, J.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Recovery of bioactive compounds from Arbutus unedo L. fruits: Comparative optimization study of maceration/microwave/ultrasound extraction techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Cadi, H.; El Cadi, A.; Kounnoun, A.; Oulad El Majdoub, Y.; Palma Lovillo, M.; Brigui, J.; Dugo, P.; Mondello, L.; Cacciola, F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arabian J. Chem. 2020, 13, 6299–6311. [Google Scholar] [CrossRef]
- Salem, I.B.; Ouesleti, S.; Mabrouk, Y.; Landolsi, A.; Saidi, M.; Boulilla, A. Exploring the nutraceutical potential and biological activities of Arbutus unedo L. (Ericaceae) fruits. Ind. Crops Prod. 2018, 122, 726–731. [Google Scholar] [CrossRef]
- Macchioni, V.; Santarelli, V.; Carbone, K. Phytochemical profile, antiradical capacity and α-glucosidase inhibitory potential of wild Arbutus unedo L. fruits from Central Italy: A chemometric approach. Plants 2020, 9, 1785. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of different enriched phenolic extracts of wild fruits from Northeastern Portugal: A comparative study. Plants Foods Hum. Nutr. 2014, 69, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, B.M.; Sánchez-Moreno, C.; de Ancos, B.; Cortes Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, M.; Tardío, J. Wild Arbutus unedo L. and Rubus ulmifolius Schott fruits are underutilized sources of valuable bioactive compounds with antioxidant capacity. Fruits 2014, 69, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Res. Int. 2011, 44, 1401–1407. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Matias, A.; Duarte, C.M.M.; Bronze, M.R. High-pressure CO2 assisted extraction as a tool to increase phenolic content of strawberry-tree (Arbutus unedo) extracts. J. CO2 Util. 2018, 27, 73–80. [Google Scholar] [CrossRef]
- Orak, H.H.; Aktas, T.; Yagar, H.; Isbilir, S.S.; Ekinci, N.; Sahin, F.H. Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (Arbutus unedo L.) fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, H.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Ennahli, S.; Messaoudi, Z.; Hanine, H. Exploring antioxidant activity, organic acid, and phenolic composition in strawberry tree fruits (Arbutus unedo L.) growing in Morocco. Plants 2020, 9, 167. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Takwa, S.; Caleja, C.; Barreira, J.C.M.; Sokovic, M.; Achour, L.; Barros, L.; Ferreira, I.C.F.R. Arbutus unedo L. and Ocimum basilicum L. as sources of natural preservatives for food industry: A case study using loaf bread. LWT Food Sci. Technol. 2018, 88, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Masmoudi, M.; Ammar, I.; Ghribi, H.; Attia, H. Physicochemical, radical scavenging activity and sensory properties of a soft cheese fortified with Arbutus unedo L. extract. Food Biosci. 2020, 35, 100579. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 2019, 275, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Morcuende, D.; Ventanas, S.; Estévez, M. Application of natural antioxidants from strawberry tree (Arbutus unedo L.) and dog rose (Rosa canina L.) to Frankfurters subjected to refrigerates storage. J. Integr. Agric. 2013, 12, 1972–1981. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Armenteros, M.; Morcuende, D. Mediterranean berries as inhibitors of lipid oxidation in porcine burger patties subjected to cooking and chilled storage. J. Integr. Agric. 2013, 12, 1982–1992. [Google Scholar] [CrossRef]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef]
- Erdogan, G.; Uysal, T. Characterization of antioxidant properties of strawberry tree (Arbutus unedo L.) and trace elements determination. J. Res. Pharm. 2020, 24, 774–785. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Asmaa, N.; Abdelaziz, G.; Boulanouar, B.; Carbonell-Barrachina, A.A.; Cano-Lamadrid, M.; Noguera-Artiaga, L. Chemical composition, antioxidant activitiy and mineral content of Arbutus unedo (leaves and fruits). J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1335–1339. [Google Scholar] [CrossRef]
- Zlabur, J.S.; Bogdanovic, S.; Voca, S.; Babojelic, M.S. Biological potential of fruit and leaves of strawberry tree (Arbutus unedo L.) from Croatia. Molecules 2020, 25, 5102. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Kachkoul, R.; Squalli Housseini, T.; Mohim, M.; el Habbani, R.; Miyah, Y.; Lahrichi, A. Chemical compounds as well as antioxidant and litholytic activities of Arbutus unedo L. leaves against calcium oxalate stones. J. Integr. Med. 2019, 17, 430–437. [Google Scholar] [CrossRef]
- Nenadis, N.; Llorens, L.; Koufogianni, A.; Díaz, L.; Font, J.; Gonzalez, J.A.; Verdaguer, D. Interactive effects of UV radiation and reduced precipitation on the seasonal leaf phenolic content/composition and the antioxidant activity of naturally growing Arbutus unedo plants. J. Photochem. Photobiol. B Biol. 2015, 153, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Guendouze-Bouchefa, N.; Madani, K.; Chibane, M.; Boulekbache-Makhlouf, L.; Hauchard, D.; Kiendrebeogo, M.; Stevigny, C.; Okusa, P.N.; Duez, P. Phenolic compounds, antioxidant and antibacterial activities of three Ericaceae from Algeria. Ind. Crops Prod. 2015, 70, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, R.; Sá, O.; Pereira, E.; Aguiar, C.; Baptista, P.; Pereira, J.A. Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Ind. Crops Prod. 2012, 37, 473–478. [Google Scholar] [CrossRef]
- Jurica, K.; Gobin, I.; Kremer, D.; Cepo, D.V.; Grubesic, R.J.; Karaconji, I.B.; Kosalec, I. Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxid 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Jardim, C.; Macedo, D.; Figueira, I.; Dobson, G.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Menezes, R.; Santos, C.N. (Poly)phenol metabolites from Arbutus unedo leaves protect yeast from oxidative injury by activation of antioxidant and protein clearance pathways. J. Funct. Foods 2017, 32, 333–346. [Google Scholar] [CrossRef]
- Erkekoglou, I.; Nenadis, N.; Samara, E.; Mantzouridou, F.T. Functional teas from the leaves of Arbutus unedo: Phenolic content, antioxidant activity and detection of efficient radical scavengers. Plants Foods Hum. Nutr. 2017, 72, 176–183. [Google Scholar] [CrossRef]
- Jurica, K.; Brcic Karaconji, I.; Mikolic, A.; Milojhovic-Opsenica, D.; Benkovic, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Tariba Lovakovic, B.; Lazarus, M.; Brcic Karaconji, I.; Jurica, K.; Zivkovic Semren, T.; Lusic, D.; Brajenovic, N.; Pelaic, Z.; Pizent, A. Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dess, M.A. Antioxidant profile of strawberry tree honey and its marker homgentistic acid in several models of oxidative strees. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Djabou, N.; Dib, M.E.A.; Allali, H.; Benderb, A.; Kamal, M.A.; Ghalem, S.; Tabti, B. Evaluation of antioxidant and antimicrobial activities of the phenolic composition of Algerian Arbutus unedo L. roots. Pharmacogn. J. 2013, 5, 275–280. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Serra, A.T.; Matias, A.A.; Duarte, C.M.M.; Bronze, M.R. Supercritical fluid extraction of Arbutus unedo distillate residues—Impact of process conditions on antiproliferative response of extracts. J. CO2 Util. 2020, 37, 29–38. [Google Scholar] [CrossRef]
- Oliveira, I.; Nunes, A.; Lima, A.; Borralho, P.; Rodrigues, C.; Ferreira, R.B.; Ribeiro, A.C. New lectins from mediterranean flora. Activity against HT29 colon cancer cells. Int. J. Molec. Sci. 2019, 20, 3059. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Fei, Z.; Cui, J.; Miao, B.; Lu, Y.; Wu, J. Biosynthesis of copper oxide nanoparticles and their in vitro cytotoxicity towards nasopharynx cancer (KB cells) cell lines. Int. J. Pharmacol. 2018, 14, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Assemian, I.C.C.; Abrini, J.; Dakka, N. In vitro antiproliferative activity of selected medicinal plants from the North-West of Morocco on several cancer cell lines. Eur. J. Integr. Med. 2018, 18, 23–29. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Pistollato, F.; Zhang, J.J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Bompadre, S.; et al. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-κβ signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J. Funct. Foods 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Forni, C.; Frattarelli, A.; Lentini, A.; Beninati, S.; Lucioli, S.; Caboni, E. Assessment of the antiproliferative activity on murine melanoma cells of extracts from elicited cell suspensions of strawberry, strawberry tree, blackberry and red raspberry. Plant Biosyst. 2016, 150, 1233–1239. [Google Scholar] [CrossRef]
- Cappadone, C.; Mandrone, M.; Chiocchio, I.; Sanna, C.; Malucelli, E.; Bassi, V.; Picone, G.; Poli, F. Antitumor potential and phytochemical profile of plants from Sardinia (Italy), a hotspot for biodiversity in the Mediterranean Basin. Plants 2020, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Merhfour, F.Z.; Legssyer, A.; Mekhfi, H.; Maallem, S.; Ziyyat, A. Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea hirsuta. Pharmazie 2007, 62, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Merhfour, F.Z.; Ziyyat, A.; Aziz, M.; Legssyer, A.; Mekhfi, H. Antidiabetic effect of some medicinal plants of Oriental Morocco in neonatal non-insulin-dependent diabetes mellitus rats. Hum. Exp. Toxicol. 2010, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Jaradat, N.; Fichtali, I.; Ouedrhiri, W.; Jodeh, S.; Ayesh, S.; Cherrah, Y.; Faouzi, M.E.A. Separation, identification, and antidiabetic activity of catechin isolated from Arbutus unedo L. plant roots. Plants 2018, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Mariotto, S.; Esposito, E.; di Paola, R.; Ciampa, A.; Mazzon, E.; de Prati, A.C.; Darra, E.; Vincenzi, S.; Cucinotta, G.; Caminiti, R.; et al. Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice. Pharmacol. Res. 2008, 57, 110–124. [Google Scholar] [CrossRef]
- Mariotto, S.; Ciampa, A.R.; Carcereri De Prati, A.; Darra, E.; Vincenzi, S.; Sega, M.; Cavalieri, E.; Shoji, K.; Suzuki, H. Aqueous extract of Arbutus unedo inhibits STAT1 activation in human breast cancer cell line MDA-MB-231 and human fibroblasts through SHP2 activation. Med. Chem. 2008, 4, 219–228. [Google Scholar] [CrossRef]
- Legssyer, A.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Herrenknecht, C.; Roumy, V.; Fourneau, C.; Laurens, A.; Hoerter, J.; Fischmeister, R. Tannins and catechin gallate mediate the vasorelaxant effect of Arbutus unedo on the rat isolated aorta. Phytother. Res. 2004, 18, 889–894. [Google Scholar] [CrossRef]
- Mekhfi, H.; El Haouari, M.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother. Res. 2006, 20, 135–139. [Google Scholar] [CrossRef]
- El Haouari, M.; López, J.J.; Mekhfi, H.; Rosado, J.A.; Salido, G.M. Antiaggregant effects of Arbutus unedo extracts in human platelets. J. Ethnopharmacol. 2007, 113, 325–331. [Google Scholar] [CrossRef]
- Dimkic, I.; Gobin, I.; Begic, G.; Antic, D.R.; Ristivojevic, P.; Jurica, K.; Beric, T.; Lozo, J.; Abram, M.; Stankovic, S. Antibacterial activity of herbal extracts towards uropathogenic Enterococcus isolates as a natural approach in control of urinary tract infections. J. Herb. Med. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Asmae, E.O.; Amina, C.H.; Hakima, S.; Abdellatif, H.; Abdeslam, E.; Saad, I.; Mohamed, L.; Mohammed, I. Extra- and intracellular antimycobaterial activity of Arbutus unedo L. Afr. J. Microbiol. Res. 2012, 6, 1283–1290. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomater. 2017, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Kachkoul, R.; Sqalli Houssaini, T.; El Habbani, R.; Miyah, Y.; Mohim, M.; Lahrichi, A.; Sidi Mohammed Ben Abdellah, U. Phytochemical screening and inhibitory activity of oxalocalcic crystallization of Arbutus unedo L. leaves. Heliyon 2018, 4, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurica, K.; Benkovic, V.; Sikiric, S.; Kopjar, N.; Brcic Karaconji, I. Liver function and DNA integrity in hepatocytes of rats evaluated after treatments with strawberry tree (Arbutus unedo L.) water leaf extract and arbutin. Drug Chem. Toxicol. 2020, 43, 127–137. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefis for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, D. Oak trees (Qeuercus spp.) as a source of extracts with biological activities: A narrative review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Yussof, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Trivedi, S.; Patel, K.; Belgamwar, V.; Wadher, K. Functional polysaccharide lentinan: Role in anti-cancer therapies and management of carcinomas. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100045. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Bankole, H.A.; Fatai, A.A.; Saibu, G.M.; Wusu, A.D. Genus Aloe as sources of antidiabetic, antihyperglycemic and hypoglycemic agents: A review. S. Afr. J. Bot. 2022, 147, 1070–1077. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Antony, P.J.; Lana, M.J.M.; da Silva, B.F.X.; Oliveira, R.V.; Jothi, G.; Hariharan, G.; Mohana, T.; Gan, R.Y.; Gurgel, R.Q.; et al. Natural products modulating interleukins and other inflammatory mediators in tumor-bearing animals: A systematic review. Phytomed. 2022, 100, 154038. [Google Scholar] [CrossRef]
- Ibarz-Blanch, N.; Morales, D.; Calvo, E.; Ros-Medina, L.; Muguerza, B.; Bravo, F.I.; Suárez, M. Role of chrononutrition in the antihypertensive effects of natural bioactive compounds. Nutrients 2022, 14, 1920. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Angiolella, L.; Sacchetti, G.; Efferth, T. Antimicrobial and antioxidant activities of natural compounds. Evid. Based Complem. Altern. Med. 2018, 2018, 1945179. [Google Scholar] [CrossRef] [PubMed]
- Modrackova, N.; Vlkova, E.; Tejnecky, V.; Schwab, C.; Neuzil-Bunesova, V. Bifidobacterium β-glucosidase activity and fermentation of dietary plant glucosides is species and strain specific. Microorganisms 2020, 8, 839. [Google Scholar] [CrossRef]
- Rudeekulthamrong, P.; Kaulpiboon, J. Optimization of amylomaltase for the synthesis of α-arbutin derivatives as tyrosinase inhibitors. Carbohydr. Res. 2020, 494, 108078. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gómez-Candela, C.; Smidt, H.; Marín, F.R.; Soler-Rivas, C. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-D-glucan-enriched extract obtained from Lentinula edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Gatea, F. Correlations between microbiota bioactivity and bioavailability of functional compounds: A mini-review. Biomedicine 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-indsutrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed]
| Arbutus Species | Geographical Distribution | Main Parts of Interest | References |
|---|---|---|---|
| A. unedo | Europe (South, West, Central), Africa (North-East), Asia (East), Canary Islands, Ireland | Fruits, leaves, roots, honeys | [1,2] |
| A. andrachne | Europe (South-East, East), Asia (West) | Fruits, leaves | [3,4] |
| A. canariensis | Canary Islands | Fruits | [5] |
| A. pavarii | Lybia | Fruits, honeys, bark | [6] |
| A. arizonica | USA, Mexico | Fruits | [2] |
| A. madrensis | Mexico | - | [2,7] |
| A. menziesii | USA, Mexico, Canada | Fruits | [8] |
| A. occidentalis | Mexico | - | [2] |
| A. tessellata | Mexico | - | [9] |
| A. xalapensis | USA, Mexico, Nicaragua, El Salvador, Guatemala, Honduras | Bark, wood | [10] |
| Arbutus × andrachnoides (A. unedo × A. andrachne) | Greece, Turkey, Cyprus | Fruits | [11] |
| Arbutus × androsterilis (A. unedo × A. canariensis) | Canary Islands | Fruits | [5] |
| Part | Main/Traditional Uses | Tested Biological Activities | Potential Health Implications | References |
|---|---|---|---|---|
| Fruit | Food, beverages, medicinal | Antioxidant, hypoglycemic, antimicrobial, antiproliferative | Diabetes, hypertension, metabolic syndrome, cancer | [14,15] |
| Leaves | Infusions, medicinal | Antioxidant, anti-inflammatory, hypoglycemic, antimicrobial, antiviral, antiproliferative, antihypertensive, anti-urolithiasis, antileishmanial | Diabetes, hypertension, metbolic syndrome, viral infections (papilloma virus), urolithiasis, leishmaniasis | [16,17] |
| Flower | Medicinal | Antioxidant | Oxidative stress-related disorders | [18] |
| Honey | Food, medicinal | Antioxidant, anti-inflammatory, antimicrobial, antiproliferative, antihypertensive | Hypertension, metabolic syndrome, cancer | [19,20,21,22] |
| Bark/wood | Firewood, tools, ornamental | Antioxidant | Oxidative stress-related disorders | [23] |
| Root | Medicinal, red dye (textile industry) | Antioxidant, hypoglycemic, antimicrobial, antihypertensive | Diabetes, hypertension, metabolic syndrome | [24,25,26,27] |
| Activity | Raw Material | Experimental Model | Bioactive Compound(s) | Extraction Method | Reference |
|---|---|---|---|---|---|
| Antioxidant | Fruits | Radical scavenging assays | Phenolic compounds | Delipidation + Ultrasound-assisted extraction (Ethyl acetate; 80% methanol) | [29] |
| 80% ethanol; 80% methanol extraction | [30] | ||||
| 80% methanol extraction | [31] | ||||
| 80 and 100% methanol extraction | [32] | ||||
| Phenolic compounds (gallic acid, cyanidin 3-glucoside), ascorbic acid | - | [33] | |||
| Phenolic compounds, fatty acids, α-tocopherol | 96% ethanol extraction | [34] | |||
| Galloyl hexoside, 5-O-galloylquinic acid | Supercritical-CO2 extraction (40, 55, 70 °C) | [35] | |||
| Radical scavenging and β-carotene bleaching assays | Phenolic compunds, ascorbic acid | Ethanol extraction | [36] | ||
| Phenolic compounds (gallocatechol, catechin) | Ethanol extraction (Ultra-turrax homogeneization, 25 °C) | [37] | |||
| Radical scavenging, β-carotene bleaching and lipid peroxidation inhibition assays | Phenolic compounds, ascorbic acids, tocopherols, carotenoids | - | [38] | ||
| Radical scavenging, β-carotene and crocin bleaching assays | Phenolic compounds, carbohydrates | Maceration (20–90 °C), microwave- (50–145 °C) and ultrosound-assisted (30–35 °C) extraction (ethanol–water mixtures) | [28] | ||
| Radical scavenging, β-carotene bleaching and lipid peroxidation inhibition assays (bread fortification) | Catechin | 23% ethanol extraction | [39] | ||
| Radical scavenging assays (yoghurt fortification) | Anthocyanins | Acidified water extraction | [14] | ||
| Radical scavenging assays (cheese fortification) | Phenolic compounds | Water infusion | [40] | ||
| Radical scavenging assays (wafers fortification) | Anthocyanins | 80% acidified ethanol (90 °C) | [41] | ||
| Food oxidative stability (sausages fortification) | Phenolic compounds | Ethanol extraction | [42] | ||
| Lipid oxidation (limpet pâté) | Water extraction | [15] | |||
| Lipid oxidation (pork burgers) | - | Ethanol extraction | [43] | ||
| Protein oxidation (pork burgers) | Ethanol extraction | [44] | |||
| Fruits, leaves | Radical scavenging assays | Phenolic compounds | 80% methanol extraction | [45] | |
| Ultrasound-assisted extraction (70% ethanol) | [46] | ||||
| Ultrasound-assisted extraction (80% methanol) | [47] | ||||
| Phenolic compounds, ascorbic acid, β-carotene | 80% ethanol | [48] | |||
| Human erythrocytes | Phenolic compounds | Water infusion (100 °C) | [49] | ||
| Fruits, flowers | Radical scavenging and β-carotene bleaching assays | Water, ethanol and methanol extraction | [18] | ||
| Leaves | Radical scavenging assays | Delipidation + hot 80% ethanol extraction or water infusion (100 °C) | [50] | ||
| Ultrasound-assisted extraction (50% methanol) | [51] | ||||
| Ethanol, methanol, diethyl ether extraction | [34] | ||||
| Delipidation + methanol extraction | [52] | ||||
| Water infusion (100 °C) | [53] | ||||
| Arbutin, phenolic compounds (hydroquinone) | Ethanol, water extraction (50 °C) | [54] | |||
| - | Ethanol maceration | [17] | |||
| Radical scavenging and β-carotene bleaching assays | Phenolic compounds, iridoids | Maceration, Soxhlet and ultrasound-assisted extraction (ethanol) | [55] | ||
| Radical scavenging assays and inhibition of β-carotene peroxidation | Phenolic compounds | Water maceration | [56] | ||
| Radical scavenging assays, Saccharomyces cerevisiae strains | 50% Ethanol extraction, in vitro digestion | [57] | |||
| Radical scavenging, crocin-bleaching and liposome-accelerated oxidation assays (teas) | Phenolic compounds (galloylquinic acid derivatives and myricitrin) | Addition of boiling water (decoction) (100 °C) | [58] | ||
| Human peripheral blood lymphocytes | Ultrasound-assisted extraction (water) | [59] | |||
| Honeys | Radical scavenging assays | Phenolic compounds | - | [60] | |
| [22] | |||||
| Phenolic compounds (arbutin) | Amberlite resin purification and methanol extraction | [21] | |||
| - | [61] | ||||
| Radical scavenging, cholesterol, liposome oxidation assays | Homogentistic acid | [62] | |||
| Wood, stalks, leaves | Radical scavenging assays | Phenolic compounds | 60% acetone, 95% ethanol extraction | [23] | |
| Roots | Phenolic compounds (catechin) | Ethyl acetate or methanol extraction (100 °C) | [63] | ||
| Phenolic compounds (flavonoids) | High-pressure solvent extraction (water, methanol, ethyl acetate, dichloromethane) | [26] | |||
| Antiproliferative/antitumoral | Fruits | Human breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460), colon carcinoma (HCT-15), cervical carcinoma (HeLa) and hepatocellular carcinoma (HepG2) cells | Phenolic compounds | 80 and 100% methanol extraction | [32] |
| Fruit residues (after fermentation and distillation) | Human colorectal adenocarcinoma (HT29) | Phenolic compounds, fatty acids esters, terpenes | Supercritical-CO2 extraction (35, 45, 55 °C), 50% ethanol extraction, Soxhlet extraction (hexane) | [64] | |
| Leaves | Proteins (lectins) | Protein extraction | [65] | ||
| Human epithelial carcinoma cells (KB) | - | Hot water extraction (80 °C) | [66] | ||
| Human cervical epithelial carcinoma (HeLa), human epidermioid carcinoma (A431), human malignant melanoma (A375) cells | Ethanol maceration | [17] | |||
| Human embryonal rhabdomyosarcoma cancerous cells (RD), rat embryonal rhabdomyosarcoma cancerous cells (L20B), monkey kidney cancerous cells (Vero) | Phenolic compounds | Hexane, ethanol and methanol extraction | [67] | ||
| Honeys | Human colon carcinoma (HCT-116) and LoVo cells | Phenolic compounds, proteins | - | [19] | |
| Phenolic compounds (phenolic acids, flavonols) | [68] | ||||
| Phenolic compounds | [19,69] | ||||
| Axillary buds, leaves fragments | Murine B6-F10 melanoma cells | Anthocyanins | Cell culture (MS medium, B5 medium) | [70] | |
| Whole plant | Human osteosarcoma cells (U2OS) | Phenolic compounds (arbutin) | Ultrasound-assisted extraction (50% methanol) | [71] | |
| Hypoglycemic | Fruits | α-glucosidase inhibition test | Phenolic compounds | 80% methanol extraction | [31] |
| Fruits, leaves | α-glucosidase and α-amylase inhibition tests | Phenolic compounds, iridoids | Maceration, Soxhlet and ultrasound-assisted extraction (ethanol) | [55] | |
| Roots | C57BL/6JRj mice, Sprague–Dawley rats | - | Hot water extraction (100 °C) | [25] | |
| Wistar rats | [72] | ||||
| [73] | |||||
| α-glucosidase inhibition test | Catechin | Pressurized liquid extraction (water, 100 °C, 10 MPa) | [74] | ||
| Anti-inflammatory/immune-modulatory | Leaves | Human red blood cells | Phenolic compounds | Water maceration | [56] |
| Human alveolar epithelial (A549/8) cells, human leukemya monocyes (THP-1) and CD mice | Methanol extraction | [75] | |||
| Human breast cancer (MDA-MB-231) cells and human fibroblasts | [76] | ||||
| Honeys | Hyaluronidase inhibition assay | Phenolic compounds (arbutin) | Amberlite resin-purification and methanol extraction | [21] | |
| Antihypertensive/vasodilatory | Leaves | Aorta isolation from Wistar rats | Tannins and catechin gallate | Water and methanol extraction; tannins precipitation by caffeine addition | [77] |
| Platelet aggregation | Tannins | Hot water (100 °C), methanol and ethyl acetate extraction; tannins precipitation by caffeine addition | [78] | ||
| - | Hot water (100 °C), diethly ether, ethly acetate extraction | [79] | |||
| Roots | Aorta isolation from Wistar rats | Hot water extraction (100 °C) | [27] | ||
| Antimicrobial | Fruits | Microbiological analyses | Phenolic compounds | 80% ethanol extraction | [30] |
| Microbiological analyses (limpet pâté) | Water extraction | [15] | |||
| Leaves | Microbiological analyses | Water infusion (100 °C) | [53] | ||
| Delipidation + methanol extraction | [52] | ||||
| Ultrasound-assisted extraction (50% ethanol) | [80] | ||||
| Phenolic compounds (hydroquinone, arbutin) | Ethanol, water extraction (50 °C) | [54] | |||
| - | Ethanol and hot water extraction (100 °C) | [81] | |||
| Microbiological analyses (silver nanoparticles) | Hot water extraction (100 °C) | [82] | |||
| Roots | Microbiological analyses | Phenolic compounds | Methanol and water extraction | [24] | |
| Phenolic compounds (flavonoids) | High-pressure solvent extraction (water, methanol, ethyl acetate, dichloromethane) | [26] | |||
| Honeys | Amberlite resin purification and methanol extraction | [21] | |||
| Pentane, dichloromethane, butanol extraction | [20] | ||||
| Antiviral | Leaves | Anti-herpes simplex virus type-1 assay | - | Ethanol maceration | [17] |
| Antileishmanial | Leaves | Anti-promastigote activity assays | Phenolic compounds | Methanol, ethanol and hexane maceration | [16] |
| Anti-urolithiasis | Leaves | Inhibition of calcium oxalate’s crystallization | Phenolic compounds, tannins, saponins, sterols, polyterpenes, alkaloids | Water and ethanol extraction (100 °C) | [50,83] |
| Evaluated Activity | Cell Model | Tested Concentration(s) | ST Administered Product | Reference |
|---|---|---|---|---|
| Antioxidant | Human erythrocytes | 50, 75, 100 µg/mL | Fruit aqueous extract | [49] |
| 0.4, 0.8, 1.6 mg/mL | Leaves aqueous extract | |||
| Saccharomyces cerevisiae | 250 µg gallic acid-equivalents/mL | Leaved hydroethanolic extract (after in vitro digestion) | [57] | |
| Human peripheral blood lymphocytes | 11.4, 200, 400 µg/mL | Leaves aqueous extract | [59] | |
| Antiproliferative/antitumoral | MCF-7, NCI-H460, HCT-15, HeLa, HepG2 | 25–400 µg/mL | Fruit methanolic extract | [32] |
| HT29 | 0.125–4 mg/mL | Fruit (residue) supercritical, hydroethanolic and hexane extracts | [64] | |
| KB | 0.05, 0.1, 0.2 mg/mL | Leaves aqueous extract | [66] | |
| HeLa, A431, A375 | 1.56–200 µg/mL | Leaves ethanolic extract | [17] | |
| RD, L20B, Vero | 3.5–250 µg/mL | Leaves ethanolic, methanolic and hexane extracts | [67] | |
| HCT-116 | 3–20 mg/mL | Honeys | [19,68,69] | |
| LoVo | 10–60 mg/mL | |||
| U202 | 50, 100 µg/mL | Hydromethanolic whole plant extracts | [71] | |
| Anti-inflammatory/immune–modulatory | Human red blood cells | 50–500 µg/mL | Leaves aqueous extract | [56] |
| A549/8, THP-1 | 0.9–59.2 µg gallic acid-equivalents/mL | Leaves methanolic extract | [75] | |
| MDA-MB-231 | 0.02–29.6 µg gallic acid-equivalents/mL | [76] | ||
| Human fibroblasts | 3.7–14.8 µg gallic acid-equivalents/mL |
| Evaluated Activity | Animal Model | Dosage | Administration | ST Administered Product | Reference |
|---|---|---|---|---|---|
| Hypoglycemic | Sprague–Dawley rats (males and females) | 0.3 and 2 g/kg | Daily at 10:00 h a.m., 6 weeks, intragastric gavage | Root aqueous extract | [25] |
| Wistar rats (males) | 0.5 g/kg | 30 min before glucose administration, oral administration | [72] | ||
| Wistar rats (males and females) | 0.15 g/kg | [73] | |||
| Anti-inflammatory/immune modulatory | CD mice (males) | 0.02 g (gallic-acid equivalents)/kg | 1 h before carragenan and saline administration, oral administration | Leaves methanolic extract | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, D. Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities. Foods 2022, 11, 3838. https://doi.org/10.3390/foods11233838
Morales D. Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities. Foods. 2022; 11(23):3838. https://doi.org/10.3390/foods11233838
Chicago/Turabian StyleMorales, Diego. 2022. "Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities" Foods 11, no. 23: 3838. https://doi.org/10.3390/foods11233838
APA StyleMorales, D. (2022). Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities. Foods, 11(23), 3838. https://doi.org/10.3390/foods11233838