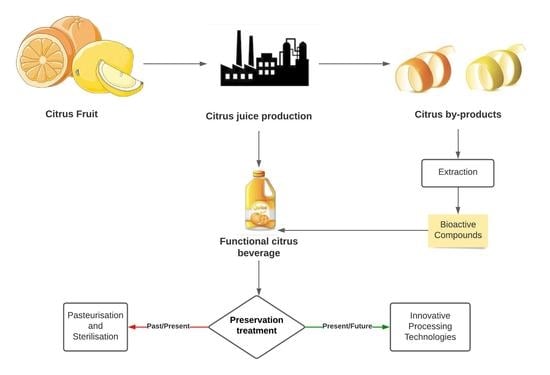
Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends
Abstract
:1. Introduction
2. Functional Citrus-Based Beverages
New Opportunities for Citrus Based-Beverages
3. Beverage Processing Technologies
3.1. Microwave Heating Treatment
3.2. Ohmic Heating Treatment
3.3. Ultrasound Treatment
3.4. High-Pressure Processing
3.5. Pulsed Eletric Field
4. Impact of IPT on Bioactive Compounds
4.1. Phenolic Compounds
4.2. Vitamin C
4.3. Carotenoids
5. Impact of IPT on Microbial Contamination, Shelf-Life, and Sensory Parameters
6. The Consumers’ Perspective
7. Trends and Innovations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative Hurdle Technologies for the Preservation of Functional Fruit Juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods: Commercial Trends, Research, and Health Implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and Challenges for Functional and Medicinal Beverages: Current and Future Trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Agricultural and Food Industry By-Products: Source of Bioactive Components for Functional Beverages. In Nutrients in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 543–589. [Google Scholar]
- Cong, L.; Bremer, P.; Mirosa, M. Functional Beverages in Selected Countries of Asia Pacific Region: A Review. Beverages 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Grand View Research. Sports Drink Market Size, Share, Growth, Industry Trends Report; Grand View Research: San Francisco, CA, USA.
- Rampersaud, G.C.; Valim, M.F. 100% Citrus Juice: Nutritional Contribution, Dietary Benefits, and Association with Anthropometric Measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota Modulation and Effects on Metabolic Biomarkers by Orange Juice: A Controlled Clinical Trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M. Effects of Hesperidin in Orange Juice on Blood and Pulse Pressures in Mildly Hypertensive Individuals: A Randomized Controlled Trial (Citrus Study). Eur. J. Nutr. 2021, 60, 1277–1288. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.; Rahman, R.; Islam, S.M.; Khan, M.S. A Review on Mechanisms and Commercial Aspects of Food Preservation and Processing. Agric. Food Secur. 2017, 6, 1–22. [Google Scholar] [CrossRef]
- Song, Q.; Li, R.; Song, X.; Clausen, M.P.; Orlien, V.; Giacalone, D. The Effect of High-Pressure Processing on Sensory Quality and Consumer Acceptability of Fruit Juices and Smoothies: A Review. Food Res. Int. 2022, 157, 111250. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 248. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to Conventional Thermal Treatments in Fruit-Juice Processing. Part 1: Techniques and Applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef]
- Puiggròs, F.; Muguerza, B.; Arola-Arnal, A.; Aragonès, G.; Suárez-Garcia, S.; Bladé, C.; Arola, L.; Suárez, M. Functional Beverages. In Innovative Technologies in Beverage Processing Spain; Wiley Online Library: New York, NY, USA, 2017. [Google Scholar]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Cilla, A.; Garcia-Llatas, G.; Lagarda, M.J.; Barberá, R.; Alegría, A. Development of Functional Beverages: The Case of Plant Sterol-Enriched Milk-Based Fruit Beverages. Funct. Med. Beverages 2019, 11, 285–312. [Google Scholar] [CrossRef]
- Raizel, R.; Coqueiro, A.Y.; Bonvini, A.; Tirapegui, J. Sports and Energy Drinks: Aspects to Consider. In Sports and Energy Drinks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–37. [Google Scholar]
- Jeyaraman, M.M.; Abou-Setta, A.M.; Grant, L.; Farshidfar, F.; Copstein, L.; Lys, J.; Gottschalk, T.; Desautels, D.; Czaykowski, P.; Pitz, M. Dairy Product Consumption and Development of Cancer: An Overview of Reviews. BMJ Open 2019, 9, e023625. [Google Scholar] [CrossRef] [Green Version]
- Moser, S.E.; Shin, J.-E.; Kasturi, P.; Hamaker, B.R.; Ferruzzi, M.G.; Bordenave, N. Formulation of Orange Juice with Dietary Fibers Enhances Bioaccessibility of Orange Flavonoids in Juice but Limits Their Ability to Inhibit in Vitro Glucose Transport. J. Agric. Food Chem. 2020, 68, 9387–9397. [Google Scholar] [CrossRef]
- Morales De la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Application of Novel Processing Methods for Greater Retention of Functional Compounds in Fruit-Based Beverages. Beverages 2016, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Rajauria, G.; Tiwari, B.K. Fruit Juices: An Overview. Fruit Juices 2018, 3–13. [Google Scholar]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- FAOSTAT Statistic Database Online; FAOSTAT: Rome, Italy. Available online: https://www.fao.org/faostat/en/#home (accessed on 17 October 2022).
- Žuntar, I.; Petric, Z.; Bursać Kovačević, D.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus Phytochemical with Health-promoting Properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikeh, E.I.; Omoregie, E.S.; Oviasogie, F.E.; Oriakhi, K. Phytochemical, Antimicrobial, and Antioxidant Activities of Different Citrus Juice Concentrates. Food Sci. Nutr. 2016, 4, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Koch, P.; Mishra, P. Optimization of Debittering and Deacidification Parameters for Pomelo Juice and Assessment of Juice Quality. J. Food Sci. Technol. 2020, 57, 4726–4732. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Fernandes, M.J.; Bernardes, J.P.; Miguel, M.G. Citrus as a Component of the Mediterranean Diet. J. Spat. Organ. Dyn. 2016, 4, 289–304. [Google Scholar]
- Uçan, F.; Ağçam, E.; Akyildiz, A. Bioactive Compounds and Quality Parameters of Natural Cloudy Lemon Juices. J. Food Sci. Technol. 2016, 53, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Jos, J.; Lid, V.; Mart, R.; Melgarejo, P.; Hern, F.; Legua, P. Physico-Chemical Attributes of Lemon Fruits as Affected by Growing Substrate and Rootstock. Foods 2022, 11, 2487. [Google Scholar]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In Vitro Availability of Flavonoids and Other Phenolics in Orange Juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Al-Kindy, S.M.Z.; Abdulnour, A.O.; Al-Rasbi, M.M. Determination of Sugar and Mineral Contents in some Omani Fruits. Sultan Qaboos Univ. J. Sci. [SQUJS] 2001, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, J.; Shan, Y.; Can, G.U.O.; Lian, H.E.; Zhang, L.; Wei, L.; Liang, Y.; Zhong, B. Effect of Harvest Time on the Chemical Composition and Antioxidant Capacity of Gannan Navel Orange Citrus sinensis L. Osbeck ‘Newhall’Juice. J. Integr. Agric. 2022, 21, 261–272. [Google Scholar] [CrossRef]
- Vavoura, M.V.; Karabagias, I.K.; Kosma, I.S.; Badeka, A.V.; Kontominas, M.G. Characterization and Differentiation of Fresh Orange Juice Variety Based on Conventional Physicochemical Parameters, Flavonoids, and Volatile Compounds Using Chemometrics. Molecules 2022, 27, 6166. [Google Scholar] [CrossRef]
- Ahmed, S.; Rattanpal, H.S.; Gul, K.; Dar, R.A.; Sharma, A. Chemical Composition, Antioxidant Activity and GC-MS Analysis of Juice and Peel Oil of Grapefruit Varieties Cultivated in India. J. Integr. Agric. 2019, 18, 1634–1642. [Google Scholar] [CrossRef]
- La Cava, E.L.M.; Sgroppo, S.C. Evolution during Refrigerated Storage of Bioactive Compounds Andquality Characteristics of Grapefruit [Citrus paradisi (Macf.)] Juice Treated with UV-C Light. LWT 2015, 63, 1325–1333. [Google Scholar] [CrossRef]
- Sicari, V.; Giuffrè, T.M.; Zappia, A.M.; And Poiana, M. Physical Chemical Properties and Antioxidant Capacities of Grapefruit Juice (Citrus paradisi) Extracted from Two Different Varieties. Int. Food Res. J. 2018, 25, 1978–1984. [Google Scholar]
- Kelebek, H. Sugars, Organic Acids, Phenolic Compositions and Antioxidant Activity of Grapefruit (Citrus paradisi) Cultivars Grown in Turkey. Ind. Crop. Prod. 2010, 32, 269–274. [Google Scholar] [CrossRef]
- Anticona, M.; Fayos, M.-C.; Esteve, M.-J.; Frigola, A.; Blesa, J.; Lopez-Malo, D. Differentiation of Juice of Mandarin-like Hybrids Based on Physicochemical Characteristics, Bioactive Compounds, and Antioxidant Capacity. Eur. Food Res. Technol. 2022, 248, 2253–2262. [Google Scholar] [CrossRef]
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The Effects of Cultivar and Harvest Date on Functional Properties of Juice and Cloudy Juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Sicari, V.; Pellicanò, T.M. Phytochemical Properties and Antioxidant Potential from Citrus Bergamia, Risso (Bergamot) Juice Extracted from Three Different Cultivars. J. Appl. Bot. Food Qual. 2016, 89, 171–175. [Google Scholar] [CrossRef]
- Cautela, D.; Laratta, B.; Santelli, F.; Trifirò, A.; Servillo, L.; Castaldo, D. Estimating Bergamot Juice Adulteration of Lemon Juice by High-Performance Liquid Chromatography (HPLC) Analysis of Flavanone Glycosides. J. Agric. Food Chem. 2008, 56, 5407–5414. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.W.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Chemical Composition and Sensory Profile of Pomelo (Citrus grandis (L.) Osbeck) Juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Hossain, M.; Rai, D.K. Waste/by-Product Utilisations; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products-A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Szczepańska, J.; Danielczuk, J.; Zawada, K. The Development and Consumer Acceptance of Functional Fruit-Herbal Beverages. Foods 2020, 9, 1819. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus by-Products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Food Waste and By-Product Valorization as an Integrated Approach with Zero Waste: Future Challenges. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 569–596. [Google Scholar]
- Tromp, R.H.; de Kruif, C.G.; van Eijk, M.; Rolin, C. On the Mechanism of Stabilisation of Acidified Milk Drinks by Pectin. Food Hydrocoll. 2004, 18, 565–572. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Dennis-Wall, J.C.; Burns, A.M.; Solch, R.J.; Ukhanova, M.; Dahl, W.J.; Christman, M.C.; Boileau, T.; Brauchla, M.; Shin, J.-E.; Nieves, C.; et al. A Beverage Containing Orange Pomace Improves Laxation and Modulates the Microbiome in Healthy Adults: A Randomised, Blinded, Controlled Trial. J. Funct. Foods 2019, 60, 103438. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.M. Thermosonication Process for Optimal Functional Properties in Carrot Juice Containing Orange Peel and Pulp Extracts. Food Chem. 2018, 245, 79–88. [Google Scholar] [CrossRef]
- Afkhami, R.; Goli, M.; Keramat, J. Functional Orange Juice Enriched with Encapsulated Polyphenolic Extract of Lime Waste and Hesperidin. Int. J. Food Sci. Technol. 2018, 53, 634–643. [Google Scholar] [CrossRef]
- Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Beverages Based on Second Quality Citrus Fruits and Maqui Berry, a Source of Bioactive (Poly) Phenols: Sorting out Urine Metabolites upon a Longitudinal Study. Nutrients 2021, 13, 312. [Google Scholar] [CrossRef]
- Harsha, H.; Aarti, S. Quality Evaluation of Herbal Juice Developed from Traditional Indian Medicinal Plants Using Citrus Limetta as Base. J. Nutr. Food Sci. 2015, 5, 1. [Google Scholar]
- Yekeler, F.Z.; Ozyurek, H.; Tamer, C.E. A Functional Beverage: Lemonade. Int. J. Nutr. Food Eng. 2013, 7, 617–620. [Google Scholar]
- Leporini, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.M.; Reitano, A.; Dugay, A.; Deguin, B.; Tundis, R. Citrus× Clementina Hort. Juice Enriched with Its by-Products (Peels and Leaves): Chemical Composition, in Vitro Bioactivity, and Impact of Processing. Antioxidants 2020, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Bento, R.; Pagán, E.; Berdejo, D.; de Carvalho, R.J.; García-Embid, S.; Maggi, F.; Magnani, M.; de Souza, E.L.; García-Gonzalo, D.; Pagán, R. Chitosan Nanoemulsions of Cold-Pressed Orange Essential Oil to Preserve Fruit Juices. Int. J. Food Microbiol. 2020, 331, 108786. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel Technologies to Improve Food Safety and Quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Jin, S.; Guo, C.; Lu, Y.; Zhang, R.; Wang, Z.; Jin, M. Comparison of Microwave and Conventional Heating Methods in Carbonization of Polyacrylonitrile-Based Stabilized Fibers at Different Temperature Measured by an in-Situ Process Temperature Control Ring. Polym. Degrad. Stab. 2017, 140, 32–41. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Moreno-Vilet, L.; Villanueva-Rodríguez, S.J. Current Status of Emerging Food Processing Technologies in Latin America: Novel Non-Thermal Processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Rajauria, G.; O’Donnell, C.P.; Tiwari, B.K. Emerging Food Processing Technologies and Factors Impacting Their Industrial Adoption. Crit. Rev. Food Sci. Nutr. 2019, 59, 3082–3101. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Cardozo, T.S.F.; Couto, S.M.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Pimentel, T.C.; Freitas, M.Q.; Raices, R.S.L.; et al. Effects of Microwave Heating on the Chemical Composition and Bioactivity of Orange Juice-Milk Beverages. Food Chem. 2021, 345, 128746. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability 2020, 12, 5556. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Oliveira, A.; Ribeiro, T.B.; Ribeiro, S.; Nunes, C.; Gómez-García, R.; Nunes, J.; Pintado, M. Impact of Extraction Process in Non-Compliant ‘Bravo de Esmolfe’ Apples towards the Development of Natural Antioxidant Extracts. Appl. Sci. 2021, 11, 5916. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Zhang, J.-J.; Li, Y.; Meng, X.; Li, H.-B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma Sanguineum Fruit: Optimization and Identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Kim, S.-S.; Kang, D.-H. Effect of PH for Inactivation of Escherichia Coli O157: H7, Salmonella Typhimurium and Listeria Monocytogenes in Orange Juice by Ohmic Heating. LWT-Food Sci. Technol. 2015, 62, 83–88. [Google Scholar] [CrossRef]
- Darvishi, H.; Hosainpour, A.; Nargesi, F.; Khoshtaghaza, M.H.; Torang, H. Ohmic Processing: Temperature Dependent Electrical Conductivities of Lemon Juice. Mod. Appl. Sci. 2011, 5, 209. [Google Scholar] [CrossRef] [Green Version]
- Piette, G.; Buteau, M.L.; De Halleux, D.; Chiu, L.; Raymond, Y.; Ramaswamy, H.S.; Dostie, M. Ohmic Cooking of Processed Meats and Its Effects on Product Quality. J. Food Sci. 2004, 69, fep71–fep78. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Baysal, T.; Icier, F.; Yildiz, H.; Demirdoven, A.; Bozkurt, H. Processing of Fruits and Fruit Juices by Novel Electrotechnologies. Food Eng. Rev. 2012, 4, 68–87. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A.K. Ohmic Heating: Concept and Applications—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2338–2351. [Google Scholar] [CrossRef]
- Chandrapala, J.; Leong, T. Ultrasonic Processing for Dairy Applications: Recent Advances. Food Eng. Rev. 2015, 7, 143–158. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Tahi, A.A.; Sousa, S.; Madani, K.; Silva, C.L.M.; Miller, F.A. Ultrasound and Heat Treatment Effects on Staphylococcus Aureus Cell Viability in Orange Juice. Ultrason. Sonochem. 2021, 78, 105743. [Google Scholar] [CrossRef]
- Mohamed, M.E.A.; Eissa, A.H.A. Pulsed Electric Fields for Food Processing Technology. Struct. Funct. Food Eng. 2012, 11, 275–306. [Google Scholar]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed Electric Field Treatment of Citrus Fruits: Improvement of Juice and Polyphenols Extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of Thermal and Non-Thermal Treatments on the Color of Citrus Juice: A Review Effect of Thermal and Non-Thermal Treatments on the Color of Citrus. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.L.; Welti-Chanes, J.; Alzamora, S.M. Hurdle Technology in Fruit Processing. Annu. Rev. Food Sci. Technol. 2011, 2, 447–465. [Google Scholar] [CrossRef]
- Tewari, G.; Juneja, V. Advances in Thermal and Non-Thermal Food Preservation. Int. J. Dairy Technol. 2009, 62, 285–286. [Google Scholar]
- Kubo, M.T.K.; Augusto, P.E.D.; Cristianini, M. Effect of High Pressure Homogenization (HPH) on the Physical Stability of Tomato Juice. Food Res. Int. 2013, 51, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in Phenolic Acids and Antioxidant Activity of Navel Orange at Different Growth Stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef]
- Esteve, M.J.; Frigola, A. The Effects of Thermal and Non-Thermal Processing on Vitamin C, Carotenoids, Phenolic Compounds and Total Antioxidant Capacity in Orange Juice. Tree For. Sci. Biotechnol. 2008, 2, 128–131. [Google Scholar]
- Li, A.; Li, S.; Zhang, Y.; Xu, X.; Chen, Y.; Li, H. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [Green Version]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and Nobiletin Induce G1 Cell Cycle Arrest but Not Apoptosis in Human Breast and Colon Cancer Cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chen, W.-J.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Tangeretin Induces Cell-Cycle G1 Arrest through Inhibiting Cyclin-Dependent Kinases 2 and 4 Activities as Well as Elevating Cdk Inhibitors P21 and P27 in Human Colorectal Carcinoma Cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Sakib, S.A.; Mahmud, S.; Khan, Z.; Islam, M.N.; Sakib, M.A.; Bin Emran, T.; Simal-Gandara, J. Identification of Potential Phytochemicals from Citrus Limon against Main Protease of SARS-CoV-2: Molecular Docking, Molecular Dynamic Simulations and Quantum Computations. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Gour, A.; Manhas, D.; Bag, S.; Gorain, B.; Nandi, U. Flavonoids as Potential Phytotherapeutics to Combat Cytokine Storm in SARS-CoV-2. Phyther. Res. 2021, 35, 4258–4283. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Cheng, F.-J.; Huynh, T.-K.; Yang, C.-S.; Hu, D.-W.; Shen, Y.-C.; Tu, C.-Y.; Wu, Y.-C.; Tang, C.-H.; Huang, W.-C.; Chen, Y. Hesperidin Is a Potential Inhibitor against SARS-CoV-2 Infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic Potential of Natural Compounds in Inflammation and Chronic Venous Insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of Novel Technologies on Polyphenols during Food Processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Thermal Treatment and Storage on the Stability of Organic Acids and the Functional Value of Grapefruit Juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Min-Tze, L.; Karim, A.A. Sonication Improves Kasturi Lime (Citrus microcarpa) Juice Quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of Ultrasound Treatments on Quality of Grapefruit Juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O.; Cano, M.P. Impact of High Pressure and Pulsed Electric Fields on Bioactive Compounds and Antioxidant Activity of Orange Juice in Comparison with Traditional Thermal Processing. J. Agric. Food Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef]
- Plaza, L.; Sánchez-Moreno, C.; De Ancos, B.; Elez-Martínez, P.; Martín-Belloso, O.; Cano, M.P. Carotenoid and Flavanone Content during Refrigerated Storage of Orange Juice Processed by High-Pressure, Pulsed Electric Fields and Low Pasteurization. LWT-Food Sci. Technol. 2011, 44, 834–839. [Google Scholar] [CrossRef]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Navarro, J.L.; Meléndez-Martínez, A.J. High-Pressure Homogenization as Compared to Pasteurization as a Sustainable Approach to Obtain Mandarin Juices with Improved Bioaccessibility of Carotenoids and Flavonoids. J. Clean. Prod. 2020, 262, 121325. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of Vitamin C in Citrus Juice Concentrates during Storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of Innovative Technologies on the Content of Vitamin C and Its Bioavailability from Processed Fruit and Vegetable Products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Vikram, V.B.; Ramesh, M.N.; Prapulla, S.G. Thermal Degradation Kinetics of Nutrients in Orange Juice Heated by Electromagnetic and Conventional Methods. J. Food Eng. 2005, 69, 31–40. [Google Scholar] [CrossRef]
- Bor Gé Czi, G.; Rk Horvá Th, M.; Kaszab, T.; Alemany, G.G. No Major Differences Found between the Effects of Microwave-Based and Conventional Heat Treatment Methods on Two Different Liquid Foods. PLoS ONE 2013, 8, e53720. [Google Scholar] [CrossRef] [Green Version]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Comparative Shelf Life Study and Vitamin C Loss Kinetics in Pasteurised and High Pressure Processed Reconstituted Orange Juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- Kolašinac, S.M.; Stevanović, Z.P.D.; Kilibarda, S.N.; Kostić, A. Carotenoids: New Applications of “Old” Pigments. Phyton 2021, 90, 1041–1062. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of Carotenoids in Orange Juice during Microwave Heating. LWT-Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Achir, N.; Dhuique-Mayer, C.; Hadjal, T.; Madani, K.; Pain, J.P.; Dornier, M. Pasteurization of Citrus Juices with Ohmic Heating to Preserve the Carotenoid Profile; Elsevier: Amsterdam, The Netherlands, 2016; Volume 33, ISBN 0467614482. [Google Scholar]
- Uckoo, R.M.; Jayaprakasha, G.K.; Somerville, J.A.; Balasubramaniam, V.M.; Pinarte, M.; Patil, B.S. High Pressure Processing Controls Microbial Growth and Minimally Alters the Levels of Health Promoting Compounds in Grapefruit (Citrus paradisi Macfad) Juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 7–14. [Google Scholar] [CrossRef]
- Cheng, C.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the Effects of Novel Processing Technologies and Conventional Thermal Pasteurisation on the Nutritional Quality and Aroma of Mandarin (Citrus unshiu) Juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102425. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Van Impe, J.; Smet, C.; Tiwari, B.; Greiner, R.; Ojha, S.; Stulić, V.; Vukušić, T.; Režek Jambrak, A. State of the Art of Nonthermal and Thermal Processing for Inactivation of Micro-organisms. J. Appl. Microbiol. 2018, 125, 16–35. [Google Scholar] [CrossRef] [Green Version]
- Elez-Martínez, P.; Soliva-Fortuny, R.C.; Martín-Belloso, O. Comparative Study on Shelf Life of Orange Juice Processed by High Intensity Pulsed Electric Fields or Heat Treatment. Eur. Food Res. Technol. 2006, 222, 321–329. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed Electric Fields as an Alternative to Thermal Processing for Preservation of Nutritive and Physicochemical Properties of Beverages: A Review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Valero, M.; Recrosio, N.; Saura, D.; Muñoz, N.; Martí, N.; Lizama, V. Effects of Ultrasonic Treatments in Orange Juice Processing. J. Food Eng. 2007, 80, 509–516. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Wang, M.-S.; Liu, Z.-W.; Han, Z.; Zhang, Z.-H.; Hong, J.; Jabbar, S. A Potential of Ultrasound on Minerals, Micro-Organisms, Phenolic Compounds and Colouring Pigments of Grapefruit Juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Ferrer, C.; Rodrigo, D.; Pina, M.C.; Klein, G.; Rodrigo, M.; Martínez, A. The Monte Carlo Simulation Is Used to Establish the Most Influential Parameters on the Final Load of Pulsed Electric Fields E. coli Cells. Food Control 2007, 18, 934–938. [Google Scholar] [CrossRef]
- Uemura, K.; Isobe, S. Developing a New Apparatus for Inactivating Bacillus Subtilis Spore in Orange Juice with a High Electric Field AC under Pressurized Conditions. J. Food Eng. 2003, 56, 325–329. [Google Scholar] [CrossRef]
- WANG, L.; PAN, J.; XIE, H.; YANG, Y.; ZHOU, D.; ZHU, Z. Pasteurization of Fruit Juices of Different PH Values by Combined High Hydrostatic Pressure and Carbon Dioxide. J. Food Prot. 2012, 75, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Aadil, R.M.; Zeng, X.-A.; Jabbar, S.; Nazir, A.; Mann, A.A.; Khan, M.K.I.; Abdullah, A.; Ramzan, A. Quality Evaluation of Grapefruit Juice by Thermal and High-Pressure Processing Treatment. Pak. J. Agric. Res. 2017, 30, 249. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Knol, J.J.; Quataert, M.C.J.; Vervoort, L.; Van Der Plancken, I.; Hendrickx, M.E.; Matser, A.M. Comparing Equivalent Thermal, High Pressure and Pulsed Electric Field Processes for Mild Pasteurization of Orange Juice. Part I: Impact on Overall Quality Attributes. Innov. Food Sci. Emerg. Technol. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Guerrouj, K.; Sánchez-Rubio, M.; Taboada-Rodríguez, A.; Cava-Roda, R.M.; Marín-Iniesta, F. Sonication at Mild Temperatures Enhances Bioactive Compounds and Microbiological Quality of Orange Juice. Food Bioprod. Process. 2016, 99, 20–28. [Google Scholar] [CrossRef]
- Patil, S.; Bourke, P.; Kelly, B.; Frías, J.M.; Cullen, P.J. The Effects of Acid Adaptation on Escherichia Coli Inactivation Using Power Ultrasound. Innov. Food Sci. Emerg. Technol. 2009, 10, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Cardello, A.V.; Schutz, H.G.; Lesher, L.L. Consumer Perceptions of Foods Processed by Innovative and Emerging Technologies: A Conjoint Analytic Study. Innov. Food Sci. Emerg. Technol. 2007, 8, 73–83. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Consumer Acceptance of Orange Juice Containing Functional Ingredients. Food Res. Int. 2004, 37, 805–814. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Kitsawad, K. Sensory Characteristics and Consumer Acceptance of Fruit Juice Containing Probiotics Beads in Thailand. AU J. Technol. 2010, 14, 33–38. [Google Scholar]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The Link between the Consumer and the Innovations in Food Product Development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef]
- Wang, E.S.-T.; Yu, J.-R. Effect of Product Attribute Beliefs of Ready-to-Drink Coffee Beverages on Consumer-Perceived Value and Repurchase Intention. Br. Food J. 2016, 118, 2963–2980. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwak, H.S. Influence of Functional Information on Consumer Liking and Consumer Perception Related to Health Claims for Blueberry Functional Beverages. Int. J. Food Sci. Technol. 2015, 50, 70–76. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Axten, L.G.; Wohlers, M.W.; Sun-Waterhouse, D. Polyphenol-rich Beverages: Insights from Sensory and Consumer Science. J. Sci. Food Agric. 2009, 89, 2356–2363. [Google Scholar] [CrossRef]
- Hung, Y.; Grunert, K.G.; Hoefkens, C.; Hieke, S.; Verbeke, W. Motivation Outweighs Ability in Explaining European Consumers’ Use of Health Claims. Food Qual. Prefer. 2017, 58, 34–44. [Google Scholar] [CrossRef]
- Siegrist, M. Factors Influencing Public Acceptance of Innovative Food Technologies and Products. Trends Food Sci. Technol. 2008, 19, 603–608. [Google Scholar] [CrossRef]
- Belyaeva, Z.; Rudawska, E.D.; Lopatkova, Y. Sustainable Business Model in Food and Beverage Industry–a Case of Western and Central and Eastern European Countries. Br. Food J. 2020, 122, 1573–1592. [Google Scholar] [CrossRef]
- Lodorfos, G.; Konstantopoulou, A.; Kostopoulos, I.; Essien, E.E. Food and Drink Industry in Europe and Sustainability Issues. In The Sustainable Marketing Concept in European SMEs; Emerald Publishing Limited: Bradford, UK, 2018; ISBN 1787540391. [Google Scholar]
- Spilimbergo, S.; Elvassore, N.; Bertucco, A. Microbial Inactivation by High-Pressure. J. Supercrit. Fluids 2002, 22, 55–63. [Google Scholar] [CrossRef]
- Coelho, P.M.; Corona, B.; ten Klooster, R.; Worrell, E. Sustainability of Reusable Packaging–Current Situation and Trends. Resour. Conserv. Recycl. X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A. Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional Milk Beverage Fortified with Phenolic Compounds Extracted from Olive Vegetation Water, and Fermented with Functional Lactic Acid Bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, R.; McDougall, G.J.; Dobson, G.; Stewart, D.; Hellström, J.; Mattila, P.; Pihlava, J.-M.; Koskela, A.; Karjalainen, R. Fortification of Blackcurrant Juice with Crowberry: Impact on Polyphenol Composition, Urinary Phenolic Metabolites, and Postprandial Glycemic Response in Healthy Subjects. J. Funct. Foods 2012, 4, 746–756. [Google Scholar] [CrossRef]
- Elegbede, J.L.; Li, M.; Jones, O.G.; Campanella, O.H.; Ferruzzi, M.G. Interactions between Flavonoid-Rich Extracts and Sodium Caseinate Modulate Protein Functionality and Flavonoid Bioaccessibility in Model Food Systems. J. Food Sci. 2018, 83, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Seymour, R.B.; Pitts, K.F.; Konczak, I.; Lundin, L. Incorporation of Functional Ingredients into Foods. Trends Food Sci. Technol. 2009, 20, 388–395. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Mirsaeedghazi, H.; Azarikia, F.; Abedinia, A. Investigation of Antimicrobial Activity of Orange and Pomegranate Peels Extracts and Their Use as a Natural Preservative in a Functional Beverage. J. Food Meas. Charact. 2021, 15, 5683–5694. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and Fruit By-Products as Sources of Bioactive Compounds. Benefits and Trends of Lactic Acid Fermentation in the Development of Novel Fruit-Based Functional Beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Saguir, F.M.; Sajur, S.A.; Pérez, M.B.; Savino, M.J.; Maturano, C. Enzymatic Activities and Fermentation Products of Lactic Acid Bacteria From Fruits and Fermented Beverages. Incidence on Food Quality. In Quality Control in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 491–528. [Google Scholar]
- Gonzalez-Molina, E.; Moreno, D.A.; Garcia-Viguera, C. Aronia-Enriched Lemon Juice: A New Highly Antioxidant Beverage. J. Agric. Food Chem. 2008, 56, 11327–11333. [Google Scholar] [CrossRef]

| Parameters | Lemon Juice | Orange Juice | Grapefruit Juice Mandarin Juice | Bergamot Juice Pomelo Pink | |||
|---|---|---|---|---|---|---|---|
| pH | 2.5–3.0 | 3.8–3.9 | 3.0–3.7 | 3.6–4.4 | 2.2–2.9 | 3.67 | |
| Sugars | Total content | -- | 84 g/L | 49.8–85.4 g/L | -- | -- | 76.5–91.0 g/L |
| Glucose | 7.5–7.9 g/L | 25.79–29.5 g/L | 22.1–24.6 g/L | 14.6 g/L | 9–13.1 g/L | 13.1 g/L | |
| Fructose | 5.4–5.8 g/L | 22.93–25.3 g/L | 22.3–26.5 g/L | 17.1 g/L | 9.7–12.6 g/L | 13.4 g/L | |
| Sucrose | 34.8–67.4 g/L | 29.5–35 g/L | 57.1 g/L | 16.8–18 g/L | 31.3–49.1 g/L | ||
| Organic Acids | Citric acid | 3.2–44.6 g/L | 16.3–23.9 g/L | -- | 1.6 g/L | 14.15 g/L | |
| Malic acid | 3.5–4.4 g/L | 0.4–3.0 g/L | -- | -- | 0.8 g/L | ||
| Succinic acid | 5.7–6.6 g/L | 0.2–0.61 g/L | -- | -- | 0.1 g/L | ||
| Ascorbic acid | 0.6 g/L | 0.1 g/L | 0.2–0.7 g/L | 0.1–0.5 g/L | 0.4–0.9 g/L | 0.2 g/L | |
| Phenolic Compounds | Hesperidin | 81.5–117.4 mg/L | 36.3–435 mg/L | 3.77–100.25 mg/L | 367–873 mg/L | -- | |
| Narirutin | 32.8–36.0 mg/L | 37.07–120.06 mg/L | 77–1450 mg/L | 18–182 mg/L | 13.8 mg/L | ||
| Eriocitrin | 16.7–391 mg/L | 1.1–6.7 mg/L | 0.1–1 mg/L | 13.4–15.6 mg/L | |||
| Neoeriocitrin | 9.7–14.3 mg/L | 103–402 mg/L | 16.0 mg/L | ||||
| Neohesperidin | 0.9–1.6 mg/L | 0.7–5.9 mg/L | 0.2–24.2 mg/L | -- | 66–554.5 mg/L | 64.8 mg/L | |
| Poncirin | 14.2–26.0 mg/L | -- | -- | ||||
| Didymin | 0.1–15 mg/L | 8–12 mg/L | 2.7–3.3 mg/L | 56.8 mg/L | -- | -- | |
| Naringin | 2.8–4.1 mg/L | 166–464.1 mg/L | -- | 97–528.2 mg/L | 600–1763.71 mg/L | ||
| Naringenin | -- | 0.1–0.3 mg/L | 24.3–31.3 mg/L | -- | -- | -- | |
| Rutin | 2.3–3.5 mg/L | -- | 12.80–14.71 mg/L | -- | -- | -- | |
| Quercetin | 1.5–2.6 mg/L | -- | -- | -- | -- | -- | |
| Ferulic acid | 0.1–0.2 mg/L | 0.03 mg/L | 14.1–26.5 mg/L | -- | -- | -- | |
| Caffeic acid | 3.4–4.7 mg/L | 0.02 mg/L | -- | -- | -- | -- | |
| p-coumaric acid | 0.1 mg/L | 0.04 mg/L | 13.7–16.30 mg/L | -- | -- | -- | |
| o-coumaric acid | 0.9–1.4 mg/L | -- | -- | -- | -- | ||
| Vannilic acid | -- | -- | 3.6–5.3 mg/L | -- | -- | -- | |
| Gallic acid | -- | -- | 3.18–4.62 mg/L | -- | -- | -- | |
| Protocatechuic acid | -- | 0.18 mg/L | 1.87–3.70 mg/L | -- | -- | -- | |
| Chlorogenic acid | 9.0–13.4 mg/L | -- | -- | -- | |||
| Vitamins | Vitamin C | 508–625.4 mg/L | 66.6–41.0 mg/L | 455–680 mg/L | 250 mg/L | 341–867 mg/L | -- |
| Minerals | Potassium | 1300 mg/L | 1000 mg/L | 74 mg/L | 1200 mg/L | 1359 mg/L | 457 mg/L |
| Iron | 4.5 mg/L | 0.4 mg/100 mL | -- | 0.5–0.6 mg/L | -- | -- | |
| Phosphorous | 100 mg/L | 50–210 mg/L | 220 mg/L | -- | -- | 120 mg/L | |
| Magnesium | 70 mg/L | 90 mg/L | 25.6 mg/100 mL | 120–133 mg/L | 92 mg/L | 289 mg/L | |
| Zinc | -- | 1 mg/L | -- | 0.3–0.4 mg/L | -- | -- | |
| Sodium | 20 mg/L | 5–30 mg/L | 8.0 mg/100 mL | 4.4–6.5 mg/L | 17 mg/L | 133 mg/L | |
| Calcium | 70 mg/L | 110 mg/L | 29.0 mg/100 mL | 39.8–43.80 mg/L | 76 mg/L | 165 mg/L | |
| References | [27,32,33,34] | [27,35,36,37,38] | [27,36,39,40,41,42] | [27,43] | [27,44,45,46] | [27,36,47] | |
| Product | Citrus by-Product | Target Function | Ref. |
|---|---|---|---|
| Carrot juice with orange peel extract | Orange peels | Increase the polyphenol content of carrot juice with orange peel extract. | [60] |
| Citrus mix juice with maqui-berry | Second Quality Citrus Fruits (orange, lemons, mandarin) | Valorisation of non-compliant citrus fruit for the development of new beverages, rich in anthocyanins and flavanones. | [62] |
| Functional Lemonade | Non-conforming lemons during harvest time | Develop a lemonade enriched with natural herb extracts (ginger, linden, and mint). | [64] |
| Orange juice with polyphenol extract from lime waste | Lime waste (Citrus latifolia) | Enrich orange juice with hesperidin from lime waste to enhance nutritional value and protect the polyphenols’ oxidation against the pasteurisation process. | [61] |
| Beverage with orange pomace addition | Orange pomace | Valorise the orange by-product by creating a functional orange juice that increases stool frequency in healthy adults. | [59] |
| Functional juice based on citrus | Citrus × clementina Hort. by-products | Ascribe antioxidant, hypoglycaemic, and hypolipidemic effects. | [65] |
| Orange juice with orange peels essential oil (EO) | Orange peels from different varieties | Prolong shelf-life of orange juice through anti-microbial activity of EO in decreasing Escherichia coli O157:H7 in. Sensory characteristics of juice with EO encapsulated in chitosan were acceptable. | [66] |
| IPT | Application Matrix | Detected Compounds | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| MH | Grapefruit juice | Total phenolic compounds (TPC) | 900 W, 30 s | ↑ Better preservation of TPC (82%) than heat treatment | [104] |
| MH | Orange juice–milk beverage | Total phenolic compounds | 2450 MHz, 65 °C, 60 s | ↑ Levels of total phenolic compounds compared to the conventional treatment (75 °C, 15 s) ↑ The MH juice showed higher ACE inhibitory activity and antioxidant activity | [72] |
| US | Lime Juice | TPC and flavonoids | 25 kHz, 20 °C, 30 to 60 min | ↑ Increase in total phenolics (263.8 up to 272.0 and 336.0 mg GAE/g), flavonoids (0.26 up to 0.30 and 0.37 mg CE/g) | [105] |
| US | Grapefruit Juice | Total phenols, flavonoids, and flavonols | 28 kHz, 20 °C | ↑ US for 90 min showed an increase in total phenols (826.27 µg/g) compared to control 0 min (757.96 µg/g), total flavonoids (603.18 µg/g) compared to control 0 min (462.27 µg/g), and total flavonols (2.94 µg/g compared to control 0 min 2.70 µg/g) | [106] |
| HPP | Orange Juice | TPC | 400 MPa, 5 min | ↑ Treated juice with HHP had a good result: (T = 4 ± 2 °C, for 7 weeks): 1.051 mg/mL compared to pasteurised juice (1.070 mg/mL); (T = 10 ± 2 °C, for 6 weeks): 1.002 mg/mL compared to pasteurised juice (0.985 mg/mL) | [94] |
| HPP | Orange Juice | Naringenin and Hesperetin | 400 MPa, 40 °C, 1 min | ↑ HP treatment led to increased naringenin (20.16%) and hesperetin (39.88%) contents | [107] |
| HPP | Orange Juice | Naringenin and Hesperetin | 400 mPa, 40 °C, 1 min | ↑ Increase in total flavanone content extracted (15.46%). Losses during the storage | [108] |
| HPP | Mandarin Juice | Total flavones and individual (Vicenin-2, apigenin d); Total flavanones and individual (naringin-d, naritutin, hesperidin, dydimin) | 150 MPa | Compared to fresh juice, processing had a positive effect on the bio-accessibility of flavonoids, although pasteurisation provided better results | [109] |
| PEF | Orange Juice | TPC | 100 µs, 30 kV/cm | ↓ Treated juice with PEF had a decrease in TPC (T = 4 ± 2 °C, for 7 weeks): 1.045 mg/mL compared to pasteurised juice (1.070 mg/mL); (T = 10 ± 2 °C, for 6 weeks): 0.941 mg/mL compared to pasteurised juice (0.985 mg/mL) | [94] |
| PEF | Orange, pomelo and lemon juice | TPC | 70 µs, 3 kV/cm | ↑ The PEF treatment increased the yield of juice (after pressing) by 25% for orange, 37% for pomelo, and 59% for lemon | [86] |
| PEF | Orange Juice | Naringenin and Hesperetin | 750 µs, 35 kV/cm | PEF juice did not show significant changes in flavanone content with regard to freshly squeezed orange juice. Losses during the storage. | [108] |
| PEF | Orange Juice | Naringenin and Hesperetin | 750 µs, 35 kV/cm | PEF treatment did not modify flavanone content. | [107] |
| IPT | Application Matrix | Detected Compounds | Mechanisms Involved | Results | Ref. |
|---|---|---|---|---|---|
| MH | Orange juice | Vitamin C | 455 W, 180 s, uncontrolled temperature | ↓ Degradation of vitamin C during MH compared to heat treatment | [112] |
| MH | Grapefruit juice | Vitamin C | 900 W, 30 s | No significant differences between MH and heat treatment | [104] |
| MH | Orange Juice | Vitamin C | 900 W, 30 s | No significant differences between MH and CH | [113] |
| US | Lime Juice | Vitamin C | 25 kHz, 20 °C | ↑ Increase in ascorbic acid content was observed compared to heat treatment | [105] |
| US | Grapefruit juice | Vitamin C | 28 kHz, 20 °C | ↑ With sonication treatment for 90 min an increase was observed (35.75 mg/ 100 mL) compared to control 0 min (27.83 mg/100 mL) | [106] |
| HPP | Orange Juice | Vitamin C | 500 MPa, 35 °C, 5 min | ↑ Better retention of ascorbic acid compared to heat treatment | [114] |
| HPP | Orange Juice | L-ascorbic acid and total Vitamin C | 400 MPa, 40 °C, 1 min | ↓ Treatments caused a significant decrease in L-AA content (7.79%)t Total Vitamin C did not exhibit any change | [107] |
| HPP | Orange Juice | Vitamin C | 4000 bars, 5 min | ↑ Treated juice with HPP had a good result: (T = 4 ± 2 °C, for 7 weeks): 42.59 mg/100 mL compared to pasteurised juice (35.58 mg/100 mL); (T = 10 ± 2 °C, for 6 weeks): 42.98 mg/100 mL compared to pasteurised juice (18.74 mg/100 mL) | [94] |
| PEF | Orange Juice | L-ascorbic acid and total Vitamin C | 750 µs, 35 kV/cm | ↓Treatments caused a significant decrease in L-AA content (7.79%) and in total Vitamin C (8.24%) | [107] |
| PEF | Orange Juice | Vitamin C | 100 µs, 30 kV/cm | ↑ Treated juice with PEF had a good result: (T = 4 ± 2 °C, for 7 weeks): 42.66 mg/100 mL compared to pasteurised juice (35.58 mg/100 mL); (T = 10 ± 2 °C, for 6 weeks): 43.03 mg/100 mL compared to pasteurised juice (18.74 mg/100 mL) | [94] |
| IPT | Application Matrix | Detected Compounds | Mechanisms Involved | Results | Ref. |
|---|---|---|---|---|---|
| OH | Grapefruit juice and Blood orange Juice | cis-violaxanthin, lutein, zeaxanthin, b-cryptoxanthin, lycopene, and β-carotene | 5 kW, 50 Hz, 0.1–3 kV·m−1 | ↓ 40–70% for xanthophylls with conventional heating; ↓ was observed using OH heating: 30% for epoxyxanthophylls and 20% for hydroxyxanthophylls | [119] |
| MH | Orange Juice | Violaxanthin, antheraxanthin, lutein, zeaxanthin, b-cryptoxanthin, α-carotene, and β-carotene | 3 kW, 2.45 GHz | ↓ At 85 °C, a decrease of approximately 50% was observed for almost all carotenoids after 1 min of MW heating | [118] |
| HPP | Orange Juice | Lutein, zeaxantin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, β-carotene | 400 MPa, 40 °C, 1 min | ↑ HPP treatment led to a carotenoid release (53.88%) | [107] |
| HPP | Grapefruit juice | All-trans-lycopene and β-carotene | 402 ± 1.9 MPa, 31.8 ± 0.5 °C, 3 min | ↑ HPP showed better results in carotenoids recovery, 14 days after storage, compared with untreated juice and thermal treated juice | [120] |
| US | Mandarin juice | Total carotenoids (β-carotene equivalents) | 50 °C, 750 W; 36 min | ↑ Total carotenoid content was higher than in the untreated samples due to US cavitation effects. | [121] |
| HPP | Orange Juice | Total carotenoids | 4000 bars for 5 min | ↑ Treated juice with HHP had a good result: (T = 4 ± 2 °C, for 7 weeks): 997.2 µg/100 g compared to pasteurised juice (913.3 µg/100 g); (T = 10 ± 2 °C, for 6 weeks): 1087.4 µg/100 g compared to pasteurised juice (854.2 µg/100 g) | [94] |
| HPP | Valencia Orange Juice | Lutein, zeaxantin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, β-carotene | 400 mPa, 40 °C, 1 min | ↑ Increase in the extractability of each individual carotenoid with regard to untreated juice and in total carotenoid content (45.19%). Furthermore, good results during storage (4 °C) were observed | [108] |
| PEF | Valencia Orange Juice | Lutein, zeaxantin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, β-carotene | 750 µs, 35 kV/cm | PEF juices did not exert any change on carotenoid content value in comparison with freshly squeezed orange juice. A good result during storage (4 °C) was observed | [108] |
| PEF | Orange Juice | Lutein, zeaxantin, α-cryptoxanthin, β-cryptoxanthin, α-carotene, β-carotene | 750 µs, 35 kV/cm | PEF treatment did not modify individual or total carotenoid content | [107] |
| PEF | Orange Juice | Total carotenoids | 100 µs, 30 kV/cm | ↑ Treated juice with PEF had good results: (T = 4 ± 2 °C, for 7 weeks): 964.2 µg/100 g compared to pasteurised juice (913.3 µg/100 g); (T = 10 ± 2 °C, for 6 weeks): 1107.8 µg/100 g compared to pasteurised juice (854.2 µg/100 g) | [94] |
| IPT | Application Matrix | Mechanisms Involved | Results | Ref. |
|---|---|---|---|---|
| PEF | Orange juice | 35 KV/cm, 4 μs pulse width, 32.5 °C | 5.1-log reduction in S. cerevisiae | [125] |
| PEF | Grapefruit juice | 1 kHz, 600 μs, 25 kV/cm, at 40 °C | 1.72-log reduction in total psychrophiles and 1.66-log reduction in yeasts and moulds | [128] |
| PEF | Orange juice | 22 and 20 kV/cm at 45 and 55 °C | 2.22-log reduction in E. coli, S. typhimurium, and non-pathogenic microbes | [129] |
| PEF | Orange juice | 16,3 KV/cm, 20 μs pulse width, 100 °C | 4-log reduction in B. subtilis | [130] |
| HPP | Orange juice | 200 MPa, 15 min; 300 MPa 15 min, at 22 °C | 5-log reduction in E. coli; 5-log reduction in Lb. plantarum | [131] |
| HPP | Grapefruit juice | 250 MPa, at 60 °C for 3 min | 2.5-log reduction in total psychrophiles and 1.1-log reduction in yeasts and moulds | [132] |
| HPP | Orange juice | 600 MPa/1 min, 17 °C | Complete inactivation of total psychrophiles, Enterobacteriaceae, E. coli, lactic acid bacteria, yeasts, and moulds | [133] |
| US | Orange juice | 24 kHz, 1, 10, 20, and 30 min, 43 to 45 °C | 1.6-log reduction in total psychrophiles and 0.9-log reduction in yeasts and moulds | [134] |
| US | Orange juice | 20 kHz, Varying amplitude levels (0.4, 7.5, 37.5 μm), 15 min, below 30 °C | 5.9-log reduction in E. coli | [135] |
| US | Orange juice | 500 kHz, 240 W for 15 min, 20 °C | 0.1- to 1.08-log reduction in total mesophilic bacteria and yeasts and moulds | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, A.A.; Magalhães, D.; Campos, D.A.; Porretta, S.; Dellapina, G.; Poli, G.; Istanbullu, Y.; Demir, S.; San Martín, Á.M.; García-Gómez, P.; et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods 2022, 11, 3859. https://doi.org/10.3390/foods11233859
Vilas-Boas AA, Magalhães D, Campos DA, Porretta S, Dellapina G, Poli G, Istanbullu Y, Demir S, San Martín ÁM, García-Gómez P, et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods. 2022; 11(23):3859. https://doi.org/10.3390/foods11233859
Chicago/Turabian StyleVilas-Boas, Ana A., Daniela Magalhães, Débora A. Campos, Sebastiano Porretta, Giovanna Dellapina, Giovanna Poli, Yildiray Istanbullu, Sema Demir, Ángel Martínez San Martín, Presentación García-Gómez, and et al. 2022. "Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends" Foods 11, no. 23: 3859. https://doi.org/10.3390/foods11233859
APA StyleVilas-Boas, A. A., Magalhães, D., Campos, D. A., Porretta, S., Dellapina, G., Poli, G., Istanbullu, Y., Demir, S., San Martín, Á. M., García-Gómez, P., Mohammed, R. S., Ibrahim, F. M., El Habbasha, E. S., & Pintado, M. (2022). Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods, 11(23), 3859. https://doi.org/10.3390/foods11233859