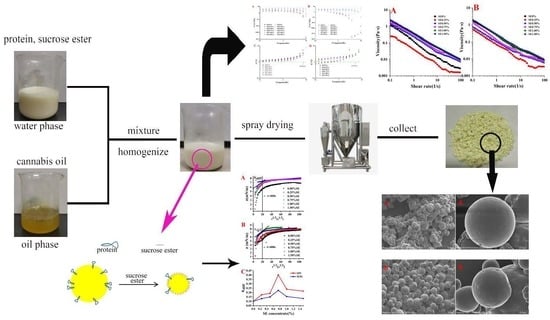
Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion
2.3. Determination of Particle Size
2.4. Determination of Zeta Potential
2.5. Observation of the Microstructure of Emulsion
2.6. Determination of Interfacial Protein Concentration
2.7. Determination of Surface Pressure of Emulsion
2.8. Shear Rheological Measurement
2.9. Determination of Viscoelastic Properties
2.10. Preparation of Microcapsules of Cannabis Oil
2.11. Observation on the Microstructure of Microcapsules
2.12. Determination of Entrapment Efficiency
2.13. Determination of Water Content and Solubility
2.14. Statistical Analysis
3. Results
3.1. Particle Size Analysis
3.2. Zeta Potential Analysis
3.3. Analysis of Interfacial Protein Loading Rate (AP%) and Interfacial Protein Concentration (Γ)
3.4. Surface Pressure Analysis
3.5. Analysis of Viscoelastic Properties
3.6. Shear Rheological Analysis
3.7. CLSM Analysis
3.8. Physicochemical Properties of Microcapsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moita, V.H.C.; Duarte, M.E.; da Silva, S.N.; Kim, S.W. Supplemental Effects of Functional Oils on the Modulation of Mucosa-Associated Microbiota, Intestinal Health, and Growth Performance of Nursery Pigs. Animals 2021, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zeng, Z.J.; Lang, Y.; Xiang, X.; Qi, G.F.; Lu, G.; Yang, X.L. Cannabis Seed Oil Alleviates Experimental Atherosclerosis by Ameliorating Vascular Inflammation in Apolipoprotein-E-Deficient Mice. J. Agric. Food Chem. 2021, 69, 9102–9110. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red Palm Oil: A Review on Processing, Health Benefits and Its Application in Food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.H.; Huang, S.J.; Liu, R.; You, J.; Xiong, S.B.; Zhang, B.J.; Rong, J.H. Storage stability and in-vitro release behavior of microcapsules incorporating fish oil by spray drying. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127234. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.B.; Liu, J.N.; Cui, Q.; Wang, X.D.; Chen, S.; Jiang, L.Z. Relationship between Molecular Flexibility and Emulsifying Properties of Soy Protein Isolate-Glucose Conjugates. J. Agric. Food Chem. 2019, 67, 4089–4097. [Google Scholar] [CrossRef]
- Fan, L.H.; Lu, Y.Q.; Ouyang, X.K.; Ling, J.H. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Dickinson, E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. Rheology and adsorption behaviour of beta-casein and beta-lactoglobulin mixed layers at the sunflower oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 669–677. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Juan, B.; Blasco-Moreno, A.; Trujillo, A.J. Ultra high-pressure homogenized emulsions stabilized by sodium caseinate: Effects of protein concentration and pressure on emulsions structure and stability. LWT-Food Sci. Technol. 2017, 76, 57–66. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Wang, Z.B.; Corredig, M. Adsorption of Soy Protein Isolate in Oil-in-Water Emulsions: Difference between Native and Spray Dried Isolate. J. Am. Oil Chem. Soc. 2011, 88, 1593–1602. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Lei, Y.J.; Luo, X.G.; Wang, Y.X.; Li, Y.; Li, B.; Liu, S.L. Impact of pH on the interaction between soybean protein isolate and oxidized bacterial cellulose at oil-water interface: Dilatational rheological and emulsifying properties. Food Hydrocoll. 2021, 115, 106609. [Google Scholar] [CrossRef]
- Liu, T.T.; Su, G.Z.; Yang, T.S. Functionalities of chitosan conjugated with lauric acid and L-carnitine and application of the modified chitosan in an oil-in-water emulsion. Food Chem. 2021, 359, 129851. [Google Scholar] [CrossRef] [PubMed]
- Du, L.Y.; Li, S.Y.; Jiang, Q.B.; Tan, Y.Q.; Liu, Y.F.; Meng, Z. Interfacial interaction of small molecular emulsifiers tea saponin and monoglyceride: Relationship to the formation and stabilization of emulsion gels. Food Hydrocoll. 2021, 117, 106737. [Google Scholar] [CrossRef]
- Zou, H.N.; Zhao, N.; Li, S.H.; Sun, S.; Dong, X.R. Physicochemical and emulsifying properties of mussel water-soluble proteins as affected by lecithin concentration. Int. J. Biol. Macromol. 2020, 163, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Marangoni, A.G. pH and stability of the alpha-gel phase in glycerol monostearate-water systems using sodium stearoyl lactylate and sodium stearate as the co-emulsifier. RSC Adv. 2015, 5, 96746–96749. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Food emulsions—Their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- Liu, Y.; Binks, B.P. A novel strategy to fabricate stable oil foams with sucrose ester surfactant. J. Colloid Interface Sci. 2021, 594, 204–216. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F.Q. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.Z.; Liu, D.L.; Long, Z.; Yang, B.; Fang, M.; Kuang, W.M.; Zhao, M.M. Effect of sucrose ester concentration on the interfacial characteristics and physical properties of sodium caseinate-stabilized oil-in-water emulsions. Food Chem. 2014, 151, 506–513. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, Y.; Liang, X.Y.; Piatko, M.; Campbell, S.; Lo, S.K.; Liu, Y.F. Synergetic interfacial adsorption of protein and low-molecular-weight emulsifiers in aerated emulsions. Food Hydrocoll. 2018, 81, 15–22. [Google Scholar] [CrossRef]
- Thanatrungrueang, N.; Harnsilawat, T. Effect of sucrose ester and carboxymethyl cellulose on physical properties of coconut milk. J. Food Sci. Technol.-Mysore 2019, 56, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, S.; Xie, F.Y.; Han, L.; Li, L.; Jiang, L.Z.; Qi, B.K.; Li, Y. Soy/whey protein isolates: Interfacial properties and effects on the stability of oil-in-water emulsions. J. Sci. Food Agric. 2021, 101, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.D.; Xu, Y.Y.; Zhang, X.N.; Wang, X.B.; Jiang, L.Z. Effect of High Pressure Treatment on Interfacial Properties, Structure and Oxidative Stability of Soy Protein Isolate-Stabilized Emulsions. J. Oleo Sci. 2019, 68, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S.; Jasniewski, J. Effect of glycosylation with gum Arabic by Maillard reaction in a liquid system on the emulsifying properties of canola protein isolate. Carbohydr. Polym. 2017, 157, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Shi, Y.; Tu, Z.C.; Zhang, L.; Wang, H.; Tian, M.; Zhang, N.H. Influence of soy lecithin concentration on the physical properties of whey protein isolate-stabilized emulsion and microcapsule formation. J. Food Eng. 2017, 207, 73–80. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.J.; Tu, Z.C.; Wang, H.; Li, R.Y.; Zhang, L.; Hang, T.; Sun, T.; Li, C. Quality evaluation of peony seed oil spray-dried in different combinations of wall materials during encapsulation and storage. J. Food Sci. Technol. 2016, 53, 2597–2605. [Google Scholar] [CrossRef] [Green Version]
- Li, C.W.; Fan, X.K.; Sun, Y.Y.; Zhou, C.Y.; Pan, D.D. Preparation, Morphology and Release of Goose Liver Oil Microcapsules. Foods 2022, 11, 1236. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Effects of different oils on the properties of soy protein isolate emulsions and gels. Food Res. Int. 2009, 42, 925–932. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Ren, X.E.; Huang, Y.C.; Huang, C.D.; Zhang, K.M. Swirling cavitation improves the emulsifying properties of commercial soy protein isolate. Ultrason. Sonochem. 2018, 42, 471–481. [Google Scholar] [CrossRef]
- Zang, Z.H.; Chou, S.R.; Geng, L.J.; Si, X.; Ding, Y.M.; Lang, Y.X.; Cui, H.J.; Gao, N.X.; Chen, Y.; Wang, M.S.; et al. Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: Color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT-Food Sci. Technol. 2021, 152, 112269. [Google Scholar] [CrossRef]
- Yang, X.Y.; Ke, C.X.; Li, L. Physicochemical, rheological and digestive characteristics of soy protein isolate gel induced by lactic acid bacteria. J. Food Eng. 2021, 292, 110243. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhang, S.; Xie, F.Y.; Zhong, M.M.; Jiang, L.Z.; Qi, B.K.; Li, Y. Effects of covalent modification with epigallocatechin-3-gallate on oleosin structure and ability to stabilize artificial oil body emulsions. Food Chem. 2021, 341, 128272. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Cunha, R.L. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: Adsorption, interfacial rheology and emulsion features. Colloids Surf. B Biointerfaces 2018, 164, 272–280. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Li, J.H.; Su, Y.J.; Gu, L.P.; Yang, Y.J.; Chang, C.H. Composite emulsifying behavior of egg white protein and rhamnolipid: Properties of the constructed high internal phase emulsions. Food Hydrocoll. 2022, 123, 160913. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qi, B.K.; Xie, F.Y.; Hu, M.; Sun, Y.F.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 11, 106391. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of different types and concentrations of fat on the physico-chemical properties of soy protein isolate gel. Food Hydrocoll. 2021, 111, 106226. [Google Scholar] [CrossRef]
- Cui, T.T.; Chen, C.J.; Jia, A.R.; Li, D.D.; Shi, Y.P.; Zhang, M.S.; Bai, X.F.; Liu, X.; Liu, C.H. Characterization and human microfold cell assay of fish oil microcapsules: Effect of spray drying and freeze-drying using konjac glucomannan (KGM)-soybean protein isolate (SPI) as wall materials. J. Funct. Food 2021, 83, 104542. [Google Scholar] [CrossRef]
- Lekshmi, R.G.K.; Tejpal, C.S.; Anas, K.K.; Chatterjee, N.S.; Mathew, S.; Ravishankar, C.N. Binary blend of maltodextrin and whey protein outperforms gum Arabic as superior wall material for squalene encapsulation. Food Hydrocoll. 2021, 121, 10676. [Google Scholar] [CrossRef]
- Zhao, G.X.; Zhu, L.J.; Yin, P.; Liu, J.; Pan, Y.Y.; Wang, S.N.; Yang, L.; Ma, T.; Liu, H.; Liu, X.Y. Mechanism of interactions between soyasaponins and soybean 7S/11S proteins. Food Chem. 2022, 368, 130857. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.X.; Yang, Z.Y.; Rao, J.J.; Chen, B.C. Modification of β-lactoglobulin by phenolic conjugations: Protein structural changes and physicochemical stabilities of stripped hemp oil-in-water emulsions stabilized by the conjugates. Food Hydrocoll. 2022, 128, 107578. [Google Scholar] [CrossRef]
- Xi, C.Y.; Sun, Z.W.; Chen, X.; Ding, X.; Zhang, T.H. Characterization of coacervation behavior between whey protein isolate and propylene glycol alginate: A morphology, spectroscopy, and thermodynamics study. Food Chem. X 2022, 15, 100402. [Google Scholar] [CrossRef] [PubMed]







| Particle Size (μm) | Zeta Potential (mV) | pH | ||||
|---|---|---|---|---|---|---|
| SE | SPI | WPI | SPI | WPI | SPI | WPI |
| 0% | 4.26 ± 0.41 b | 5.85 ± 0.16 a | −41.03 ± 2.76 a | −45.63 ± 1.02 a | 7.41 ± 0.03 a | 7.98 ± 0.03 a |
| 0.25% | 4.82 ± 0.33 a | 5.05 ± 0.05 b | −57.63 ± 1.59 b | −46.87 ± 0.95 a | 7.46 ± 0.01 b | 8.45 ± 0.01 b |
| 0.50% | 3.73 ± 0.22 c | 3.67 ± 0.29 c | −60.60 ± 1.00 c | −52.07 ± 2.02 b | 7.57 ± 0.02 c | 8.81 ± 0.02 c |
| 0.75% | 2.80 ± 0.16 d | 3.57 ± 0.12 c | −68.17 ± 0.29 d | −54.93 ± 0.97 c | 7.75 ± 0.02 d | 9.00 ± 0.02 d |
| 1.00% | 3.09 ± 0.06 d | 3.51 ± 0.08 c | −68.57 ± 0.35 d | −61.50 ± 0.27 d | 7.93 ± 0.02 e | 9.10 ± 0.02 e |
| 1.50% | 2.98 ± 0.30 d | 2.74 ± 0.18 d | −69.47 ± 2.15 d | −73.00 ± 0.69 e | 8.19 ± 0.02 f | 9.31 ± 0.02 f |
| SPI | WPI | |||||
|---|---|---|---|---|---|---|
| SE | K | n | R2 | K | n | R2 |
| 0.00% | 0.14 ± 0.00 | 0.06 ± 0.01 | 0.99 | 0.26 ± 0.00 | 0.12 ± 0.01 | 0.99 |
| 0.25% | 0.05 ± 0.00 | 0.21 ± 0.02 | 0.98 | 0.07 ± 0.00 | 0.13 ± 0.01 | 0.99 |
| 0.50% | 0.24 ± 0.00 | 0.20 ± 0.01 | 0.99 | 0.10 ± 0.00 | 0.15 ± 0.01 | 0.99 |
| 0.75% | 0.31 ± 0.00 | 0.22 ± 0.01 | 0.99 | 0.14 ± 0.00 | 0.00 ± 0.01 | 0.99 |
| 1.00% | 0.38 ± 0.00 | 0.20 ± 0.00 | 0.99 | 0.25 ± 0.00 | 0.15 ± 0.01 | 0.99 |
| 1.50% | 0.37 ± 0.00 | 0.17 ± 0.00 | 0.99 | 0.29 ± 0.00 | 0.20 ± 0.00 | 0.99 |
| SPI-SE | WPI-SE | |
|---|---|---|
| Surface oil content (g/g) | 0.56 ± 0.08 b | 0.83 ± 0.00 a |
| Encapsulation efficiency (%) | 81.33 ± 2.67 b | 72.40 ± 0.15 a |
| Moisture content (%) | 2.38 ± 0.12 b | 2.02 ± 0.20 a |
| Solubility (%) | 97.72 ± 1.92 a | 96.65 ± 1.51 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Shi, Y.; Tu, Z.; Hu, Y.; He, C. Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior. Foods 2022, 11, 3923. https://doi.org/10.3390/foods11233923
Zhang Q, Shi Y, Tu Z, Hu Y, He C. Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior. Foods. 2022; 11(23):3923. https://doi.org/10.3390/foods11233923
Chicago/Turabian StyleZhang, Qun, Yan Shi, Zongcai Tu, Yueming Hu, and Chengyan He. 2022. "Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior" Foods 11, no. 23: 3923. https://doi.org/10.3390/foods11233923
APA StyleZhang, Q., Shi, Y., Tu, Z., Hu, Y., & He, C. (2022). Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior. Foods, 11(23), 3923. https://doi.org/10.3390/foods11233923