Goji Berry (Lycium Barbarum L.) Carotenoids Enrichment through ‘Green’ Extraction Method Improves Oxidative Stability and Maintains Fatty Acids of Yak Ghee with Microwave Heating and Storage
Abstract
:1. Introduction
2. Materials and Methods
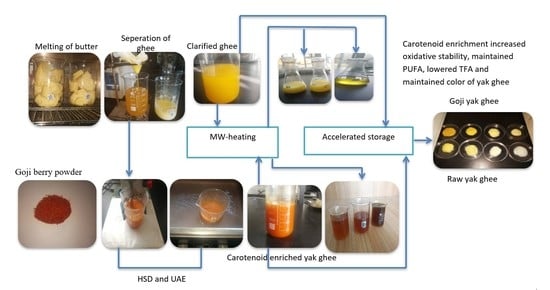
2.1. Preparation of Yak Ghee
2.2. Yield, Moisture, Solids-Not Fat (SNF), and Fat Content of Yak Ghee
2.3. Microwave Heating (MW) and Accelerated Storage
2.4. Color Assessment
2.5. Total Carotenoid Content (TCC)
2.6. Acidity Value (AV)
2.7. Peroxide Value (PV)
2.8. Thiobarbituric Acid (TBA) Test
2.9. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.10. 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Radical Scavenging Activity
2.11. Fatty Acids Analyses
2.11.1. FAME Preparation
2.11.2. GC-MS Parameters
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Yak Ghee Samples
3.2. Color Assessment
3.3. Total Carotenoid Content
3.4. Free Fatty Acids (FFA) and Oxidative Stability (AV, PV and TBA)
3.5. Radical Scavenging Activity (RSA)
3.6. Fatty Acids Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FG0 | Fresh yak ghee at 0 |
| GG0 | Goji berry carotenoid-enriched yak ghee |
| FGB15 | Fresh yak ghee heated at 180 °C for 15 min |
| FGB30v | Fresh ghee heated at 180 °C for 30 min |
| GGB15 | Goji berry ghee heated at 180 °C for 15 min |
| GGB30 | Goji berry ghee heated at 180 °C for 30 min |
| FGD30 | Goji berry ghee heated at 200 °C for 30 min |
| FG10 | Fresh yak ghee stored for 10 days |
| FG20 | Fresh yak ghee stored for 20 days |
| FG30 | Fresh yak ghee stored for 30 days |
| GG10 | Goji berry yak ghee stored for 10 days |
| GG20 | Goji berry yak ghee stored for 20 days |
| GG30 | Goji berry yak ghee stored for 30 days |
| UAE | Ultrasound-assisted extraction |
| HSD | High shear dispersion |
| PUFA | Poly unsaturated fatty acids |
| TFA | Trans fatty acids |
| MUFA | Monounsaturated fatty acids |
| SFA | Saturated fatty acids |
| CLA | Conjugated linoleic acid |
| TCC | Total carotenoid content |
| SCFA | Sort chain fatty acids |
| MCFA | Medium chain fatty acids |
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| AV | Acid value |
| PV | Peroxide value |
| TBA | Thiobarbituric acid |
References
- Sarwar, A.; Vunguturi, S.; Ferdose, A. A Study on Smoke Point and Peroxide Values of different widely used Edible Oils. Int. J. Eng. Technol. Sci. Res. 2016, 3, 271–273. [Google Scholar]
- Brühl, L. Fatty acid alterations in oils and fats during heating and frying. Eur. J. Lipid Sci. Technol. 2014, 116, 707–715. [Google Scholar] [CrossRef]
- Blasi, F.; Rocchetti, G.; Montesano, D.; Lucini, L.; Chiodelli, G.; Ghisoni, S.; Baccolo, G.; Simonetti, M.S.; Cossignani, L. Changes in extra-virgin olive oil added with Lycium barbarum L. carotenoids during frying: Chemical analyses and metabolomic approach. Food Res. Int. 2018, 105, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M. Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of. Foods 2019, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Perez-herrera, A.; Rangel-zuñiga, O.A.; Delgado-lista, J.; Marin, C.; Perez-martinez, P.; Tasset, I.; Tunez, I.; Quintana-navarro, G.M.; Lopez-segura, F.; Luque, M.D.; et al. The antioxidants in oils heated at frying temperature, whether natural or added, could protect against postprandial oxidative stress in obese people. Food Chem. 2013, 138, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- El-Shourbagy, G.A.; El-Zahar, K.M. Oxidative stability of ghee as affected by natural antioxidants extracted from food processing wastes. Ann. Agric. Sci. 2014, 59, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Kapadiya, D.B.; Aparnathi, K.D. Evaluation of commonly used herbs to enhance shelf life of ghee against oxidative deterioration. J. Food Process. Preserv. 2018, 42, e13658. [Google Scholar] [CrossRef]
- Shende, S.; Patel, S.; Arora, S.; Sharma, V. Oxidative stability of ghee incorporated with clove extracts and BHA at elevated temperatures. Int. J. Food Prop. 2014, 17, 1599–1611. [Google Scholar] [CrossRef]
- Rizzo, G.; Borrello, M.; Guccione, G.D.; Schifani, G.; Cembalo, L. Organic Food Consumption: The Relevance of the Health Attribute. Sustainability 2020, 12, 595. [Google Scholar] [CrossRef] [Green Version]
- Baria, B.; Upadhyay, N.; Singh, A.K.; Malhotra, R.K. Optimization of ‘green’ extraction of carotenoids from mango pulp using split plot design and its characterization. LWT 2019, 104, 186–194. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N. Organic solvent-free extraction of carotenoids from carrot bio- waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible vegetable oils enriched with carotenoids extracted from by- products of sea buckthorn (Hippophae rhamnoides ssp. sinensis): The investigation of some characteristic properties, oxidative stability and the effect on thermal behaviour. J. Therm. Anal. Calorim. 2019, 5, 735–747. [Google Scholar] [CrossRef]
- Covaci, E.; Senila, M.; Leopold, L.F.; Olah, N.K.; Cobzac, C.; Ivanova-Petropulos, V.; Balabanova, B.; Cadar, O.; Becze, A.; Ponta, M.; et al. Characterization of Lycium barbarum L. berry cultivated in North Macedonia: Achemometric approach. J. Berry Res. 2019, 10, 223–241. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Senizza, B.; Lucini, L.; Blasi, F. Untargeted Metabolomics to Evaluate the Stability of Extra-Virgin Olive Oil with Added Lycium barbarum Carotenoids during Storage. Foods 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Stephenson, R.C.; Ross, R.P. Carotenoids in Milk and the Potential for Dairy Based Functional Foods. Funct. Foods 2021, 10, 1263. [Google Scholar] [CrossRef]
- Lalou, S.; Ordoudi, S.A.; Mantzouridou, F.T. On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue. Foods 2021, 10, 2650. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.; Cheng, J.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Peña-serna, C.; Gómez-ramirez, B.; Zapata-lópez, N. Nutritional Aspects of Ghee Based on Lipid Composition. Pak. J. Nutr. 2019, 18, 1107–1114. [Google Scholar] [CrossRef]
- Agyare, A.N.; Liang, Q. Nutrition of yak milk fat—Focusing on milk fat globule membrane and fatty acids—Science Direct. J. Funct. Foods 2021, 83, 104404. [Google Scholar] [CrossRef]
- Evers, J.M.; Crawford, R.A.; Kissling, R.C. Determination of moisture, solids-not-fat and fat-by-difference in butter using routine methods according to ISO 8851/IDF 191—An international collaborative study and a meta-analysis. Int. Dairy J. 2003, 13, 55–65. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, H.; Wang, D.; Ma, Y.; Wang, X.; Blasi, F. Improvement for Oxidative Stability and Sensory Properties of Sunflower Oil Flavored by Huai Chrysanthemum × morifolium Ramat. Essential Oil during Accelerated Storage. Processes 2021, 9, 1199. [Google Scholar] [CrossRef]
- Koczoñ, P.; Gruczyñska, E.; Kowalski, B. Changes in the acid value of butter during storage at different temperatures as assessed by standard methods or by FT-IR spectroscopy. Am. J. Food Technol. 2008, 3, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Adjonu, R.; Zhou, Z.; Prenzler, P.D.; Ayton, J.; Blanchard, C.L. Different processing practices and the frying life of refined canola oil. Foods 2019, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- China National Standard. GB_T 35252-2017 China National Standards. Animal and vegetable fats and oils―Determination of 2-thiobarbituric acid value―Direct method. China Natl. Stand. 2017. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT35252-2017 (accessed on 15 October 2021).
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Suwarat, N.; Tungjaroenchai, W. Characteristic of Ghees Obtained from Different Post-Clarification Temperatures. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 332–334. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Alimentarius CODEX STANDARD Milk and Milk Products, 2nd ed.; Codex Alimentarius Commission: Rome, Italy, 2011; ISBN 9789251058374. [Google Scholar]
- Sserunjogi, M.L.; Abrahamsen, R.K.; Narvhus, J. A review paper: Current knowledge of ghee and related products. Int. Dairy J. 1998, 8, 677–688. [Google Scholar] [CrossRef]
- Kwak, H.S.; Ganesan, P.; Mijan, M.A. Butter, Ghee, and Cream Products. Milk Dairy Prod. Hum. Nutr. Prod. Compos. Health 2013, 18, 390–411. [Google Scholar] [CrossRef]
- Deosarkar, S.S.; Khedkar, C. Ghee; College of Dairy Technology, Pusad, India SD Kalyankar, Government College of Dairy Technology, Udgir, India ã: Udgir, India, 2016. [Google Scholar]
- Sergiu, P. The Effect of Fat Content and Fatty Acids Composition on Color and Textural Properties of Butter. Molecules 2021, 26, 4565. [Google Scholar] [CrossRef]
- Taluri, S.S.; Jafari, S.M.; Bahrami, A. Evaluation of changes in the quality of extracted oil from olive fruits stored under different temperatures and time intervals. Sci. Rep. 2019, 9, 19688. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. JFS R Concise Rev. Food Sci. 2015, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; Del Castillo, M.D. Elena Ibañez and Maria Dolores del Castillo Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, G.E.; Osman, F. Effect of Microwave Heating on Flavour Generation and Food Processing; IntechOpen: Giza, Egpt, 2012. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, T.F.; Faulkner, H.; McAuliffe, S.; O’Sullivan, M.G.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; Stanton, C.; Ross, R.P. Quality characteristics, chemical composition, and sensory properties of butter from cows on pasture versus indoor feeding systems. J. Dairy Sci. 2016, 99, 9441–9460. [Google Scholar] [CrossRef] [Green Version]
- Adadi, P.; Vasilyevna, B.N.; Krivoshapkina, E.F. Selected methods of extracting carotenoids, characterization and health concern: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Énicaud, C.P.; Chir, N.A.; Ayer, C.D.H.; Ornier, M.D.; Ohuon, P.B. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. EDP Sci. 2011, 66, 417–440. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, R.; Oliveira, I.; Vilas-boas, M.; Falcão, S.; Bento, A.; Pereira, J.A. Effect of microwave heating with different exposure times on physical and chemical parameters of olive oil. Food Chem. Toxicol. 2009, 47, 92–97. [Google Scholar] [CrossRef]
- Mohammad, S.; Hashemi, B.; Khaneghah, A.M.; Koubaa, M.; Lopez-cervantes, J.; Hossein, S.; Yousefabad, A.; Hosseini, S.F.; Karimi, M.; Motazedian, A.; et al. Novel edible oil sources: Microwave heating and chemical properties. Food Res. Int. 2016, 92, 147–153. [Google Scholar] [CrossRef]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of carotenoids in orange juice during microwave heating. LWT 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, B.D. Changes in carotenoids during processing and storage of foods. Arch. Latinoam. Nutr. 1999, 49, 38S–47S. [Google Scholar] [PubMed]
- Dongho Dongmo, F.F.; Annie, N.N.; Adélaide, D.M.; Florian, S.I.G. Effect of Heating and of Short Exposure to Sunlight on Carotenoids Content of Crude Palm Oil. J. Food Process. Technol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the lipid oxidation process of robusta green coffee beans and shelf life prediction during accelerated storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amores, G.; Virto, M. Total and free fatty acids analysis in milk and dairy fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Reitznerová, A.; Uleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 11, 1988. [Google Scholar] [CrossRef] [Green Version]
- Dostálová, J.; Hanzlík, P.; Réblová, Z.; Pokorný, J.A.N. Oxidative Changes of Vegetable Oils during Microwave Heating. Czech J. Food Sci. 2003, 23, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Uddin, I. The Coadministration of Unoxidized and Oxidized Desi Ghee Ameliorates the Toxic Effects of Thermally Oxidized Ghee in Rabbits. Hindawi J. Nutr. Metab. 2017, 2017, 4078360. [Google Scholar] [CrossRef]
- Asha, A.; Manjunatha, M.; Rekha, R.M.; Surendranath, B.; Heartwin, P.; Rao, J.; Magdaline, E.; Sinha, C. Antioxidant activities of orange peel extract in ghee (butter oil) stored at different storage temperatures. J. Food Sci. Technol. 2015, 52, 8220–8227. [Google Scholar] [CrossRef] [Green Version]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.T.; Bule, M.; Ullah, R.; Nadeem, M.; Asif, S.; Niaz, K. The antioxidant components of milk and their role in processing, ripening, and storage: Functional food. Vet. World 2019, 12, 12–33. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Carotenoids and triacylglycerols interactions during thermal oxidation of refined olive oil. Food Chem. 2011, 127, 1584–1593. [Google Scholar] [CrossRef]
- Benakmoum, A.; Abbeddou, S.; Ammouche, A. Valorisation of low quality edible oil with tomato peel waste. Food Chem. 2008, 110, 684–690. [Google Scholar] [CrossRef]
- Marquardt, S.; Barsila, S.R.; Amelchanka, S.L.; Devkota, N.R.; Kreuzer, M.; Leiber, F. Fatty acid pro fi le of ghee derived from two genotypes (cattle—Yak vs. yak) grazing different alpine Himalayan pasture sites. Anim. Prod. Sci. 2015, 58, 358–368. [Google Scholar] [CrossRef]
- Ali, A.; Mesran, H.; Bin; Latip, A.; Othman, H.; Mahmood, N. Effect of microwave heating with different exposure times on the degradation of corn oil. Int. Food Res. J. 2016, 23, 842–848. [Google Scholar]
- Topkafa, M.; Ayyildiz, H.F. Ay An implementation of central composite design: Effect of microwave and conventional heating techniques on the triglyceride composition and trans isomer formation in corn oil. Int. J. Food Prop. 2017, 20, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Afaneh, I.; Abbadi, J.; Al-Rimawi, F.; Al-Dabbas, G.; Sawalha, S. Effect of Frying Temperature and duration on the Formation of Trans Fatty Acids in Selected Fats and Oils. Am. J. Food Sci. Technol. 2017, 6, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Herzallah, S.M.; Humeid, M.A.; Al-Ismail, K.M. Effect of Heating and Processing Methods of Milk and Dairy Products on Conjugated Linoleic Acid and Trans Fatty Acid Isomer Content. J. Dairy Sci. 2005, 88, 1301–1310. [Google Scholar] [CrossRef]
- Sen, M.; Samaddar, M.; Sarkar, P.; Lal, D. Effect of high heat treatment on poly unsaturated fatty acid profile of ghee. Indian J. Dairy Sci. 2017, 70, 209–214. [Google Scholar]
- Ali, M.A.; Islam, M.A.; Hidayu, N.; Ahmadilfitri, O. Effect of heating on oxidation stability and fatty acid composition of microwave roasted groundnut seed oil. J. Food Sci. Technol. 2017, 54, 4335–4343. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, J.; Yin, J.; Li, S. Computational and experimental research on mechanism of cis/trans isomerization of oleic acid. Heliyon 2018, 4, e00768. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Physical Properties | FG0 | GG0 | |
|---|---|---|---|
| Moisture | % | 0.25 ± 0.02 | 0.17 ± 0.01 |
| Solids-not-fat (w/w) | % | 0.00 | 1.08 ± 0.02 |
| Fat Content | % | 99.50 ± 0.48 | 98.6 ± 0.2 |
| Yield | % | 78.33 ± 1.33 | 81.15 ± 1.05 |
| Fatty Acids% | FGO | GG0 | FGB15 | GGB15 | FGB30 | GGB30 | FGD30 | GGD30 | Standard Deviations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCFA | 0.96 c* | 0.90 c* | 0.99 c | 0.94 bc | 1.28 b* | 1.07 b* | 1.46 a | 1.24 a | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | 0.06 | 0.01 | 0.01 |
| MCFA | 4.14 b | 4.02 c | 6.00 a | 6.14 a | 5.99 a | 5.87 a | 5.47 a* | 5.21 b* | 0.13 | 0.05 | 0.17 | 0.26 | 0.21 | 0.18 | 0.26 | 0.21 |
| SFA | 58.48 d* | 60.50 c* | 61.80 c* | 62.84 b* | 65.51 b* | 63.50 b* | 66.05 a* | 64.33 a* | 0.45 | 0.51 | 0.39 | 0.48 | 0.71 | 0.66 | 0.53 | 0.69 |
| MUFA | 25.60 a* | 24.76 a* | 24.06 b* | 23.39 b* | 20.78 c* | 23.18 b* | 19.43 d* | 22.30 d* | 0.52 | 0.35 | 0.43 | 0.38 | 0.33 | 0.22 | 0.25 | 0.28 |
| PUFA | 8.12 a | 8.08 a | 5.73 b* | 6.91 b* | 4.01 c* | 6.07 c* | 3.53 d* | 5.15 d* | 0.16 | 0.44 | 0.20 | 0.15 | 0.18 | 0.25 | 0.20 | 0.10 |
| TFA | 8.24 d* | 6.25 d* | 8.78 c* | 6.91 c* | 9.92 b* | 7.20 b* | 11.18 a* | 7.88 a* | 0.18 | 0.22 | 0.06 | 0.11 | 0.13 | 0.18 | 0.19 | 0.14 |
| Myristic | 5.30 d* | 6.18 d* | 6.20 c | 6.51 c | 7.55 b* | 7.03 b* | 8.45 a* | 7.89 a* | 0.26 | 0.32 | 0.28 | 0.10 | 0.16 | 0.21 | 0.15 | 0.12 |
| Palmitic | 21.06 d* | 23.90 c* | 22.75 c* | 24.07 c* | 24.04 b* | 25.85 b* | 25.42 a | 26.18 a* | 0.09 | 0.16 | 0.37 | 0.30 | 0.20 | 0.20 | 0.29 | 0.23 |
| Stearic | 20.09 c* | 18.10 c* | 20.05 c* | 18.49 b* | 20.60 b* | 18.58 b* | 21.36 a* | 18.88 a* | 0.30 | 0.35 | 0.18 | 0.24 | 0.14 | 0.21 | 0.21 | 0.29 |
| Oleic | 20.59 a* | 19.24 a* | 19.02 b | 19.11 a | 17.50 c* | 18.95 b* | 16.64 d* | 18.35 c* | 0.50 | 0.41 | 0.15 | 0.26 | 0.32 | 0.18 | 0.00 | 0.05 |
| Elaidic | 6.19 c* | 4.96 c* | 6.83 c* | 5.00 c* | 8.21 b* | 5.21 b* | 9.16 a* | 5.60 a* | 0.07 | 0.10 | 0.00 | 0.01 | 0.02 | 0.04 | 0.10 | 0.06 |
| ALA | 1.47 b | 1.39 b | 1.51 a* | 1.73 a* | 0.90 c* | 1.24 c* | 0.75 d* | 1.00 d* | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| CLA | 2.81 a | 2.97 a | 2.68 b* | 2.92 a* | 2.44 c* | 2.83 b* | 2.06 d* | 2.36 c* | 0.04 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| Fatty Acids% | FGO | GG0 | FG10 | GG10 | FG20 | GG20 | FG30 | GG30 | Standard Deviations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCFA | 0.96 c* | 0.90 c* | 1.02 b* | 0.98 b* | 1.28 a* | 1.06 a* | 1.36 a* | 1.08 a* | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.03 | 0.02 | 0.01 |
| MCFA | 4.14 b | 4.02 c | 4.54 ab | 4.66 a | 4.44 ab* | 4.08 c* | 4.75 a* | 4.34 b* | 0.13 | 0.05 | 0.20 | 0.26 | 0.11 | 0.15 | 0.24 | 0.32 |
| SFA | 58.48 d* | 60.50 c* | 61.58 b | 60.98 c | 66.34 b* | 63.21 b* | 69.59 a* | 66.71 a* | 0.45 | 0.51 | 0.57 | 0.49 | 0.60 | 0.73 | 0.55 | 0.67 |
| MUFA | 25.60 a* | 24.76 a* | 23.57 b* | 24.28 a* | 20.38 c* | 23.38 b* | 17.03 d* | 21.56 c* | 0.52 | 0.35 | 0.40 | 0.48 | 0.19 | 0.21 | 0.38 | 0.44 |
| PUFA | 8.12 a | 8.08 a | 6.81 b* | 7.66 b* | 4.03 c* | 6.57 c* | 2.76 d* | 4.52 d* | 0.16 | 0.44 | 0.25 | 0.15 | 0.35 | 0.21 | 0.27 | 0.30 |
| TFA | 8.24 d* | 6.25 d* | 8.56 c* | 6.81 bc* | 9.63 b* | 7.06 ab* | 11.03 a* | 7.30 a* | 0.18 | 0.22 | 0.30 | 0.35 | 0.19 | 0.27 | 0.20 | 0.23 |
| Myristic | 5.30 d* | 6.18 d* | 5.85 c* | 6.22 c* | 6.82 b* | 6.47 b* | 8.06 a* | 7.59 a* | 0.26 | 0.32 | 0.2 | 0.24 | 0.13 | 0.17 | 0.13 | 0.19 |
| Palmitic | 21.06 c* | 23.90 d* | 23.00 b* | 24.32 c* | 24.99 a* | 25.53 b* | 25.29 a* | 26.43 a* | 0.09 | 0.16 | 0.33 | 0.26 | 0.20 | 0.23 | 0.26 | 0.21 |
| Stearic | 20.09 c* | 18.10 c* | 20.30 c* | 18.47 bc* | 21.00 b* | 18.88 b* | 21.80 a* | 19.63 a* | 0.30 | 0.35 | 0.25 | 0.11 | 0.17 | 0.21 | 0.22 | 0.31 |
| Oleic | 20.59 a* | 19.24 a* | 18.70 b* | 19.01 b* | 15.92 c* | 18.41 c* | 13.07 d* | 17.12 d* | 0.50 | 0.41 | 0.31 | 0.23 | 0.30 | 0.18 | 0.19 | 0.15 |
| Elaidic | 6.19 d* | 4.96 c* | 6.86 c* | 5.61 b* | 8.63 b* | 5.83 b* | 10.03 a* | 6.30 a* | 0.07 | 0.10 | 0.05 | 0.04 | 0.03 | 0.02 | 0.11 | 0.10 |
| ALA | 1.47 a | 1.39 b | 1.49 a | 1.42 b | 1.03 b* | 1.61 a* | 0.64 c* | 1.07 c* | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| CLA | 2.81 a | 2.97 a | 2.30 b* | 2.86 a* | 1.96 c* | 2.58 b* | 1.54 d* | 2.05 c* | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agyare, A.N.; An, C.H.; Liang, Q. Goji Berry (Lycium Barbarum L.) Carotenoids Enrichment through ‘Green’ Extraction Method Improves Oxidative Stability and Maintains Fatty Acids of Yak Ghee with Microwave Heating and Storage. Foods 2022, 11, 369. https://doi.org/10.3390/foods11030369
Agyare AN, An CH, Liang Q. Goji Berry (Lycium Barbarum L.) Carotenoids Enrichment through ‘Green’ Extraction Method Improves Oxidative Stability and Maintains Fatty Acids of Yak Ghee with Microwave Heating and Storage. Foods. 2022; 11(3):369. https://doi.org/10.3390/foods11030369
Chicago/Turabian StyleAgyare, Anita Nkansah, Chang Hong An, and Qi Liang. 2022. "Goji Berry (Lycium Barbarum L.) Carotenoids Enrichment through ‘Green’ Extraction Method Improves Oxidative Stability and Maintains Fatty Acids of Yak Ghee with Microwave Heating and Storage" Foods 11, no. 3: 369. https://doi.org/10.3390/foods11030369
APA StyleAgyare, A. N., An, C. H., & Liang, Q. (2022). Goji Berry (Lycium Barbarum L.) Carotenoids Enrichment through ‘Green’ Extraction Method Improves Oxidative Stability and Maintains Fatty Acids of Yak Ghee with Microwave Heating and Storage. Foods, 11(3), 369. https://doi.org/10.3390/foods11030369