Post-Harvest Quality Evaluation of “Soreli” Kiwifruit at Two Ripening °Brix Values from Vineyards of Different Age Under Hail Nets
Abstract
:1. Introduction
2. Materials and Methods
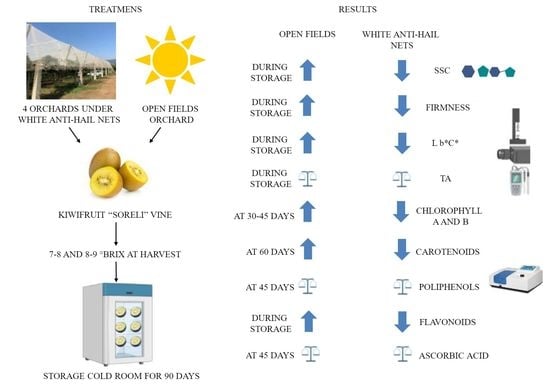
2.1. Plant Material and Experimental Design
2.2. Storage Conditions
2.3. Chemical and Physical Parameters
2.4. Bioactive Compounds Content
2.5. Statistical Analyses
3. Results
3.1. Chemical and Physical Parameters
3.2. Bioactive Compounds
4. Discussion
4.1. Chemical and Physical Parameters
4.2. Bioactive Compounds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Ortega, E.; Fita, L.; Romero, R.; López, L.; Ramis, C.; Sánchez, J.L. Numerical Simulation and Sensitivity Study of a Severe Hailstorm in Northeast Spain. Atmos. Res. 2007, 83, 225–241. [Google Scholar] [CrossRef]
- Piani, F.; Crisci, A.; De Chiara, G.; Maracchi, G.; Meneguzzo, F. Recent Trends and Climatic Perspectives of Hailstorms Frequency and Intensity in Tuscany and Central Italy. Nat. Hazards Earth Syst. Sci. 2005, 5, 217–224. [Google Scholar] [CrossRef]
- Cesari, M.; Maistrello, L.; Ganzerli, F.; Dioli, P.; Rebecchi, L.; Guidetti, R. A Pest Alien Invasion in Progress: Potential Pathways of Origin of the Brown Marmorated Stink Bug Halyomorpha Halys Populations in Italy. J. Pest. Sci. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Tacconi, G.; Paltrinieri, S.; Mejia, J.F.; Fuentealba, S.P.; Bertaccini, A.; Tosi, L.; Giacopini, A.; Mazzucchi, U.; Favaron, F.; Sella, L.; et al. Vine Decline in kiwifruit: Climate change and effect on waterlogging and phytophthora in north italy. Acta Hortic. 2015, 1096, 93–97. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Caldera, E.; Sorrenti, G.; Spinelli, F. Pathogens Associated to Kiwifruit Vine Decline in Italy. Agriculture 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Manja, K.; Aoun, M. The use of nets for tree fruit crops and their impact on the production: A review. Sci. Hortic. 2019, 246, 110–122. [Google Scholar] [CrossRef]
- Snelgar, W.P.; Hopkirk, G. Effect of Overhead Shading on Yield and Fruit Quality of Kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 2019; 63, 731–742. [Google Scholar] [CrossRef]
- Basile, B.; Romano, R.; Giaccone, M.; Barlotti, E.; Colonna, V.; Cirillo, C.; Shahak, Y.; Forlani, M. Use of photo-selective nets for hail protection of kiwifruit vines in southern Italy. Acta Hortic. 2008, 770, 185–192. [Google Scholar] [CrossRef]
- Shahak, Y. Photo-Selective Netting for Improved Performance of Horticultural Crops. A Review of Ornamental and Vegetable Studies Carried Out in Israel. Acta Hortic. 2008, 770, 161–168. [Google Scholar] [CrossRef]
- Shahak, Y.; Gussakovsky, E.; Gal, E.; Ganelevin, R. ColorNets: Crop Protection and Light-Quality Manipulation in One Technology. Acta Hortic. 2004, 659, 143–151. [Google Scholar] [CrossRef]
- Bastías, R.M.; Manfrini, L.; Corelli Grappadelli, L. Exploring the Potential Use of Photo-Selective Nets for Fruit Growth Regulation in Apple. Chil. J. Agric. Res. 2012, 72, 224–231. [Google Scholar] [CrossRef]
- Amarante, C.; Steffens, C.A.; Argenta, L. Yield and Fruit Quality of ‘Gala’ and ‘Fuji’ Apple Trees Protected by White Anti-Hail Net. Elsevier 2011, 129, 79–85. [Google Scholar] [CrossRef]
- Greer, D.; Halligan, E.A. Photosynthetic and Fluorescence Light Responses for Kiwifruit (Actinidia deliciosa) Leaves at Different Stages of Development on Vines Grown at Two Different Photon Flux Densities. Funct. Plant Biol. 2001, 28, 373–382. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of Photoselective Colour Nets on the Vegetative, Productive, and Qualitative Behaviour of Kiwifruit, Jintao Cultivar. J. Berry Res. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Hunsche, M.; Blanke, M.M.; Noga, G. Does the Microclimate under Hail Nets Influence Micromorphological Characteristics of Apple Leaves and Cuticles? J. Plant Physiol. 2010, 167, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Goffi, V.; Zampella, L.; Forniti, R.; Petriccione, M.; Botondi, R. Effects of Ozone Postharvest Treatment on Physicochemical and Qualitative Traits of Actinidia Chinensis ‘Soreli’ during Cold Storage. J. Sci. Food Agric. 2019, 99, 5654–5661. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Messina, R.; Vizzotto, G.; Testolin, R. Harvest Time and Storage of ‘Soreli’ Kiwifruit (Actinidia chinensis Planch.). Acta Hortic. 2018, 459–464. [Google Scholar] [CrossRef]
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of Antioxidant System to Postharvest Ozone Treatment in ‘Soreli’ Kiwifruit. J. Sci. Food Agric. 2020, 100, 961–968. [Google Scholar] [CrossRef]
- Goffi, V.; Modesti, M.; Forniti, R.; Botondi, R. Quality of Green (Actinidia Chinensis Var. Deliciosa ‘Hayward’) and Yellow (Actinidia chinensis Var. Chinensis ‘Soreli’) Kiwifruit during Cold Storage at 0 °C in Normal Atmosphere and with Gaseous Ozone. Acta Hortic. 2018, 1218, 473–480. [Google Scholar] [CrossRef]
- Dichio, B.; Tuzio, A.C.; Xiloyannis, C.; Rigo, G.; Lovato, R.; Comuzzo, G.; Frezza, R.; Macor, D.; Cipriani, G.; Testolin, R.; et al. The new yellow-fleshed kiwifruit (Actinidia chinensis pl.) “soreli”: Conclusions from six years of cultivation in different climatic areas. Acta Hortic. 2015, 1096, 149–154. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. Hort Science: A publication of the American Society for Horticultural Science (USA). Am. Soc. Hortic. Sci. 1992, 27, 2. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Malik, A.U.; Zora, S. Pre-Storage Application of Polyamines Improves Shelf-Life and Fruit Quality of Mango. J. Hortic. Sci. Biotechnol. 2005, 80, 363–369. [Google Scholar] [CrossRef]
- Shahak, Y.; Ratner, K.; Giller, Y.E.; Zur, N.; Or, E.; Gussakovsky, E.E.; Stern, R.; Sarig, P.; Raban, E.; Harcavi, E.; et al. Improving solar energy utilization, productivity and fruit quality in orchards and vineyards by photoselective netting. In XXVII International Horticultural Congress-IHC2006: International Symposium on Enhancing Economic and Environmental; ISHS: Bierbeek, Belgium, 2006; Volume 772, pp. 65–72. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Kührt, U.; Samietz, J.; Höhn, H.; Dorn, S. Modelling the Phenology of Codling Moth: Influence of Habitat and Thermoregulation. Agric. Ecosyst. Environ. 2006, 117, 29–38. [Google Scholar] [CrossRef]
- Allan, P.; Carlson, C. Effects of shade level on kiwifruit leaf efficiency in a marginal area. Acta Hortic. 2003, 610, 509–516. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yuan, F.-R.; He, K.-J.; Bu, F.-W. Effects of overhead shading on yield and fruit quality of kiwifruit in regions with high temperatures in summer. Acta Hortic. 2007, 753, 399–407. [Google Scholar] [CrossRef]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-Selective Hail Nets Affect Fruit Size and Quality in Hayward Kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. The Microclimate under Coloured Hailnets Affects Leaf and Fruit Temperature, Leaf Anatomy, Vegetative and Reproductive Growth as Well as Fruit Colouration in Apple. Ann. Appl. Biol. 2010, 156, 121–136. [Google Scholar] [CrossRef]
- Middleton, S.; McWaters, A. Hail Netting of Apple Orchards—Australian Experience. Compact. Fruit Tree 2002, 35, 51–55. [Google Scholar]
- Widmer, A. Light intensity and fruit quality under hail protection nets. In VII International Symposium on Orchard and Plantation Systems; ISHS: Bierbeek, Belgium, 2000; Volume 557, pp. 421–426. [Google Scholar] [CrossRef]
- Davison, R.M. Vine Factors Affecting Kiwifruit Quality and Storage Life. Orchard. 1977, 50, 161. [Google Scholar]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The Nutritional and Health Attributes of Kiwifruit: A Review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Fung, R.W.M.; Wang, S.Y.; Wang, C.Y. Transcript Levels of Antioxidative Genes and Oxygen Radical Scavenging Enzyme Activities in Chilled Zucchini Squash in Response to Superatmospheric Oxygen. Postharvest Biol. Technol. 2008, 47, 151–158. [Google Scholar] [CrossRef]
- Antognozzi, E.; Boco, M.; Famiani, F.; Palliotti, A.; Tombesi, A. Effect of different light intensity on quality and storage life of kiwifruit. Acta Hortic. 1995, 379, 483–490. [Google Scholar] [CrossRef]
- Ranjbaran, E.; Gholami, M.; Jensen, M. Changes in Phenolic Compounds, Enzymatic and Non-Enzymatic Antioxidant Properties in “Thompson Seedless” Grape after UV-C Irradiation. J. Food Process. Preserv. 2021, 45, e15965. [Google Scholar] [CrossRef]
- Zeng, J.K.; Jiang, Z.T.; Li, W.; Zhang, L.B.; Shao, Y.Z. Effects of UV-C Irradiation on Postharvest Quality and Antioxidant Properties of Wampee Fruit (Clausena lansium (Lour.) Skeels) during Cold Storage. Fruits 2020, 75, 36–43. [Google Scholar] [CrossRef]
- Severo, J.; De Oliveira, I.R.; Bott, R.; Le Bourvellec, C.; Renard, C.M.G.C.; Page, D.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C Radiation Impacts Strawberry Metabolite Content and Volatile Organic Compound Production. LWT 2017, 85, 390–393. [Google Scholar] [CrossRef]
- Olivares-Soto, H.; Bastías, R.M.; Calderón-Orellana, A.; López, M.D. Sunburn control by nets differ-entially affects the antioxidant properties of fruit peel in ‘Gala’ and ‘Fuji’apples. Hortic. Environ. Biotechnol. 2020, 61, 241–254. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, S. Bioactive Compounds in Baby Spinach (Spinacia oleracea, L.). Available online: https://pub.epsilon.slu.se/1218/ (accessed on 4 December 2021).
- Makus, D.J.; Lester, G. Effect of Soil Type, Light Intensity, and Cultivar on Leaf Nutrients in Mustard Greens. Subtrop. Plant Sci. 2002, 54, 23–28. [Google Scholar]
| Orchard 1 (OF) | kiwifruit vines in open field harvested at 7–8 °Brix (8 years) |
|---|---|
| Orchard 2 | kiwifruit vines under white net harvested at 7–8 °Brix (8 years) |
| Orchard 3 | kiwifruit vines under white net harvested at 8–9 °Brix (8 years) |
| Orchard 4 | kiwifruit vines under white net harvested at 7–8 °Brix (9 years) |
| Orchard 5 | kiwifruit vines under white net harvested at 8–9 °Brix (9 years) |
| Parameters | Treatments | 0 d | 15 d | 30 d | 45 d | 60 d | 75 d | 90 d |
|---|---|---|---|---|---|---|---|---|
| FFP (kg cm−2) | Orchard 1 (OF) | 6.98 ± 0.26 (dC) | 4.57 ± 0.30 (cCD) | 3.21 ± 0.08 (bB) | 2.95 ± 0.07 (abB) | 2.31 ± 0.03 (aB) | 1.93 ± 0.02 (aB) | 1.30 ± 0.02 (aA) |
| Orchard 2 | 5.98 ± 0.12 (dB) | 4.39 ± 0.30 (cBC) | 3.17 ± 0.11 (bB) | 2.74 ± 0.06 (abAB) | 2.33 ± 0.02 (aA) | 1.94 ± 0.04 (aB) | 1.29 ± 0.02 (aA) | |
| Orchard 3 | 4.36 ± 0.34 (cA) | 3.30 ± 0.15 (bA) | 2.87 ± 0.08 (abA) | 2.67 ± 0.05 (aA) | 2.45 ± 0.03 (abAB) | 1.72 ± 0.02 (aA) | 1.43 ± 0.02 (aA) | |
| Orchard 4 | 6.33 ± 0.21 (dBC) | 5.37 ± 0.33 (cD) | 3.54 ± 0.18 (bC) | 3.28 ± 0.24 (bC) | 2.46 ± 0.03 (aAB) | 1.93 ± 0.03 (aB) | 1.42 ± 0.02 (aA) | |
| Orchard 5 | 5.89 ± 0.18 (dB) | 4.01 ± 0.26 (cB) | 3.02 ± 0.09 (bA) | 2.61 ± 0.03 (bA) | 2.32 ± 0.04 (abB) | 1.54 ± 0.01 (aA) | 1.35 ± 0.03 (aA) | |
| SSC (°Brix) | Orchard 1 (OF) | 7.53 ± 0.27 (aAB) | 11.33 ± 0.30 (bBC) | 13.72 ± 0.31 (cD) | 14.84 ± 0.25 (cdD) | 15.2 ± 0.27 (dC) | 16.78 ± 0.26 (eC) | 17.32 ± 0.20 (eD) |
| Orchard 2 | 7.02 ± 0.12 (aA) | 10.46 ± 0.21 (bB) | 12.66 ± 0.15 (cBC) | 13.15 ± 0.18 (cdC) | 13.1 ± 0.18 (cA) | 13.87 ± 0.13 (eB) | 13.84 ± 0.14 (deB) | |
| Orchard 3 | 8.66 ± 0.09 (aB) | 13.24 ± 0.26 (bD) | 13.67 ± 0.30 (bcCD) | 13.22 ± 0.26 (bC) | 14.2 ± 023 (bcdB) | 14.27 ± 0.15 (cdB) | 14.7 ± 0.22 (dC) | |
| Orchard 4 | 7.07 ± 0.18 (aA) | 8.43 ± 0.23 (bA) | 9.99 ± 0.24 (cA) | 10 ± 0.17 (cA) | 14.2 ± 0.19 (eBC) | 13.9 0.11 (eB) | 13.9 ± 0.23 (eBC) | |
| Orchard 5 | 8.4 ± 0.19 (aB) | 11.89 ± 0.27 (bC) | 12.18 ± 0.27 (bB) | 12.06 ± 0.40 (bB) | 13.9 ± 0.27 (cAB) | 12.98 ± 0.31 (bcA) | 13 ± 0.24 (bcA) | |
| TA (mg citric | Orchard 1 (OF) | 1.52 ± 0.06 (aA) | 1.45 ± 0.15 (aA) | 1.11 ± 0.06 (aA) | 1.46 ± 0.19 (aA) | 1.38 ± 0.007 (aB) | 1.66 ± 0.1 (aA) | 1.51 ± 0.06 (aB) |
| acid/100g FW) | Orchard 2 | 1.28 ± 0.01 (bcAB) | 1.32 ± 0.05 (bA) | 0.96 ± 0.05 (aA) | 1.23 ± 0.06 (abA) | 1.19 ± 0.042 (abcAB) | 1.13 ± 0.04 (abA) | 1.03 ± 0.02 (abA) |
| Orchard 3 | 0.98 ± 0.001 (aA) | 1.02 ± 0.06 (aA) | 1.13 ± 0.009 (aA) | 1.16 ± 0.14 (aA) | 1.01 ± 0.005 (aA) | 1.16 ± 0.11 (aA) | 1.07 ± 0.08 (aAB) | |
| Orchard 4 | 1.30 ± 0.14 (abAB) | 1.37 ± 0.05 (aA) | 1.04 ± 0.03 (aA) | 1.38 ± 0.02 (aA) | 1.42 ± 0.03 (abB) | 1.66 ± 0.17 (aA) | 1.50 ± 0.13 (abB) | |
| Orchard 5 | 1.37 ± 0.10 (aAB) | 1.05 ± 0.09 (aA) | 1.10 ± 0.02 (aA) | 1.38 ± 0.09 (aA) | 1.16 ± 0.11 (aAB) | 1.04 ±0.07 (aA) | 1.01 ± 0.04 (aA) |
| Parameters | Treatments | 0 d | 15 d | 30 d | 45 d | 60 d | 75 d | 90 d |
|---|---|---|---|---|---|---|---|---|
| L | Orchard 1 (OF) | 71 ± 0.42 (cC) | 59.27 ± 0.65 (aA) | 65.94 ± 0.40 (bD) | 66.61 ± 0.35 (bC) | 59.96 ± 0.50 (aD) | 58.16 ± 0.44 (aD) | 58.79 ± 0.32 (aC) |
| Orchard 2 | 65.56 ± 0.62 (dB) | 66.03 ± 0.72 (dC) | 61.72 ± 0.39 (cC) | 49 ± 0.69 (aA) | 52.89 ± 0.45 (bBC) | 52.5 ± 0.46 (bBC) | 51.91 ± 0.36 (bB) | |
| Orchard 3 | 61.05 ± 1 (cA) | 59.17 ± 0.53 (cA) | 47.27 ± 0.76 (aA) | 49.08 ± 0.62 (abA) | 49.70 ± 0.50 (abA) | 50.32 ± 0.46 (bA) | 48.84 ± (abA) | |
| Orchard 4 | 65.15 ± 0.46 (dB) | 64.40 ± 0.33 (dBC) | 61.72 ± 0.97 (cC) | 52.73 ± 0.62 (abB) | 54.03 ± 0.44 (bC) | 52.32 ± 0.46 (abC) | 50.72 ± 0.36 (aB) | |
| Orchard 5 | 68.65 ± 0.31 (dC) | 63. 45 ± 0.37 (cB) | 50.58 ± 0.61 (aB) | 54.62 ± 0.43 (bB) | 51.67 ± 0.39 (aB) | 50.8 ± 0.41 (aAB) | 50.72 ± (aB) | |
| a* | Orchard 1 (OF) | -0.54 ± 0.04 (aB) | -0.57 ± 0.05 (aB) | -0.48 ±0.03 (aC) | -0.53 ± 0.04 (aD) | -0.57 ± 0.04 (aA) | -0.60 ± 0.04 (aA) | -0.62 ± 0.04 (aB) |
| Orchard 2 | -0.76 ± 0.06 (abB) | -0.58± 0.05 (bB) | -0.82 ± 0.06 (abB) | -0.89 ± 0.08 (aB) | -0.65 ± 0.04 (abA) | -0.65 ± 0.04(abA) | -0.62 ± 0.05 (bB) | |
| Orchard 3 | -0.76 ± 0.08 (bB) | -0.5± 0.04 (aA) | -1.08 ± 0.08 (aA) | -0.81 ± 0.06 (abBC) | -0.73 ± 0.05 (bA) | -0.62 ± 0.04 (bA) | -0.76 ± 0.05 (bB) | |
| Orchard 4 | -1.85 ± 0.13 (abA) | -0.9± 0.07 (aA) | -0.71 ± 0.06 (dBC) | -0.86 ± 0.06 (abcA) | -0.61 ± 0.04 (dA) | -0.64 ± 0.03 (bcA) | -1.23 ± 0.09 (cA) | |
| Orchard 5 | -0.73 ± 0.05 (aB) | -0.65± 0.05 (aB) | -0.7 ± 0.05 (aBC) | -0.58 ± 0.04 (aCD) | -0.67 ± 0.04 (aA) | -0.63 ± 0.04 (aA) | -0.7 ± 0.04 (aB) | |
| b* | Orchard 1 (OF) | 26.64 ± 0.29 (cdD) | 21.9 ± 0.39 (aA) | 27.04 ± 0.28 (cdC) | 29.11 ±0.26 (eC) | 26.06 ± 0.33 (cdC) | 24.3 ± 0.36 (bC) | 27.82 ± 0.35 (deC) |
| Orchard 2 | 21.18 ± 0.39 (bA) | 20.81 ± 0.41 (bA) | 25.08 ± 0.32 (cB) | 18.04 ± 0.41 (aA) | 21 ± 0.39 (bA) | 21 ± 0.34 (bA) | 21.82 ± 0.35 (bB) | |
| Orchard 3 | 21.74 ± 0.39 (cAB) | 25.21 ± 0.39 (dB) | 18.08 ± 0.54 (aA) | 19.37 ± 0.46 (abA) | 21.43 ± 0.36 (cA) | 21.32 ± 0.27 (cAB) | 20.27 ± 0.36 (bcA) | |
| Orchard 4 | 22.93 ± 0.40 (bB) | 26.9 ± 0.34 (cC) | 26.26 ± 0.54 (cBC) | 19.01 ± 0.40 (aA) | 22.14 ± 0.29 (bAB) | 22.14 ± 0.37 (bAB) | 21.33 ± 0.32 (bAB) | |
| Orchard 5 | 25.20 ± 0.33 (dC) | 26.79 ± 0.32 (eC) | 19.19 ± 0.43 (aA) | 23.27 ± 0.49 (cB) | 23.04 ±0.33(cB) | 22.4 ± 0.30 (cB) | 20.82 ± 0.31 (bAB) | |
| Chroma | Orchard 1 (OF) | 26.65 ± 0.29 (cdD) | 21.94 ± 0.39 (aA) | 27.04 ± 0.28 (cdC) | 29.12 ± 0.26 (eC) | 26.07 ± 0.33 (cC) | 24.36 ± 0.36 (bC) | 27.83 ± 0.35 (deC) |
| Orchard 2 | 21.2 ± 0.39 (bA) | 20.82 ± 0.40 (bA) | 25.1 ± 0.32 (cB) | 18.08 ± 0.41 (aA) | 21.01 ± 0.39 (bA) | 21.01 ± 0.30 (bA) | 21.95 ± 0.36 (bcA) | |
| Orchard 3 | 21.78 ± 0.38 (cAB) | 25.23 ± 0.39 (dB) | 18.15 ± 0.53 (aA) | 19.4 ± (0.46 (abA) | 21.45 ± 0.36 (cA) | 21.33 ± 0.27 (cAB) | 20.3 ± 0.36 (bcA) | |
| Orchard 4 | 23.04 ± 0.40 (cB) | 27.1 ± 0.34 (dC) | 26.31 ± 0.51 (dBC) | 19.1 ± 0.40 (aA) | 22.16 ± 0.29 (bcAB) | 22.15 ± 0.37 (bcAB) | 21.38 ± 0.32 (bAB) | |
| Orchard 5 | 25.22 ± 0.33 (dC) | 26.8 ± 0.32 (eC) | 19.22 ± 0.49 (aA) | 23.28 ± 0.43 (cB) | 23.05 ± 0.33 (cB) | 22.46 ± 0.30 (cB) | 20.84 ± 0.31 (bAB) |
| Parameters | Treatments | 0 d | 15 d | 30 d | 45 d | 60 d | 75 d | 90 d |
|---|---|---|---|---|---|---|---|---|
| Cl a | Orchard 1 (OF) | 179.6 ± 19.9 (bA) | 61.1 ± 2.78 (aA) | 58.51 ± 15.1 (aAB) | 48.97 ± 9.85 (aAB) | 68.87 ± 9.51 (aB) | 43.76 ± 1 (aC) | 43.69 ± 1.15 (aC) |
| Orchard 2 | 167.18 ± 18.06 (cA) | 73.01 ± 2.53 (bA) | 44.82 ± 18.74 (abAB) | 47.64 ± 0.40 (abAB) | 11.91 ± 0.93 (aA) | 4.86 ± 2.02 (aA) | 16.06 ± 1.47 (aB) | |
| Orchard 3 | 149.59 ± 18.94 (cA) | 60.6 ± 14.24 (bA) | 60.44 ± 0.84 (bAB) | 17.74 ± 4.34 (abA) | 11.02 ± 2.14 (aA) | 11.73 ± 1.98 (aA) | 9.36 ± 1.07 (aA) | |
| Orchard 4 | 172.82 ± 9.74 (bA) | 78.22 ± 12.67 (aA) | 78.08 ± 7.9 (aB) | 69.32 ± 16.65 (aB) | 10.85 ± 1.48 (aA) | 7.80 ± 0.89 (aA) | 3.80 ± 0.74 (aA) | |
| Orchard 5 | 122.39 ± 1.7 (eA) | 42.95 ± 2.91 (dA) | 29.56 ± 1.56 (cA) | 9.46 ± 1.86 (aA) | 17.89 ± 0.55 (abA) | 16.30 ± 3.91 (bcB) | 19.11 ± 0.82 (abB) | |
| Cl b | Orchard 1 (OF) | 260.15 ± 8.43 (bA) | 121.49 ± 0.3 (aAB) | 100.53 ± 32.87 (aA) | 87.01 ± 17.7 (aB) | 88.99 ± 38.45 (aB) | 86.54 ± 1.54 (aC) | 85.97 ± 0.78 (aD) |
| Orchard 2 | 246.97 ± 6.6 (dA) | 171.31 ± 8.71 (cBC) | 95.72 ± 22.77 (bA) | 89.48 ± 5.95 (bB) | 24.88 ± 1.76 (aA) | 20.15 ± 4.72 (aA) | 37.52 ± 2.35 (aC) | |
| Orchard 3 | 188.36 ± 9.59 (cA) | 117.05 ± 17.21 (bAB) | 132.39 ± 5.76 (bA) | 33.97 ± 8.14 (aA) | 18.03 ± 2.9 (aA) | 21.73 ± 2.52 (aA) | 17.56 ± 2.19 (aB) | |
| Orchard 4 | 159.50 ± 26.58 (bA) | 125.53 ± 13.33 (bC) | 127.74 ± 13.33 (bC) | 101.70 ± 10.47 (abB) | 27.75 ± 4.27 (aA) | 15.86 ± 2.01 (aA) | 8.06 ± 1.5 (aA) | |
| Orchard 5 | 233.57 ± 1.04 (cA) | 74.19 ± 14.67 (bA) | 57.44 ± 2.52 (abA) | 23.17 ± 1.63 (aA) | 40.50 ± 4.12 (abAB) | 34.20 ± 6.93 (abB) | 44.43 ± 1.2 (abC) | |
| CAR | Orchard 1 (OF) | 39.04 ± 4.82 (cB) | 18.05 ± 0.85 (bB) | 20.78 ± 3.29 (bB) | 19.32 ± 1.29 (bB) | 11.36 ± 0.54 (aC) | 7.21 ± 0.14 (aB) | 7.14 ± 0.17 (aC) |
| Orchard 2 | 35.72 ± 2.81(cAB) | 17.47 ± 1.85 (bB) | 16.8 ± 4.34 (bB) | 17.12 ± 0.45 (bB) | 4.79 ± 0.15 (aAB) | 4.01 ± 0.23 (aA) | 4.94 ± 0.37 (aB) | |
| Orchard 3 | 33.08 ± 0.14 (dAB) | 11.44 ± 0.69 (bA) | 14.41 ± 0.03 (cAB) | 15.67 ± 0.16 (cB) | 3.33 ± 0.25 (aA) | 4.01 ± 0.23 (cB) | 2.31 ± 0.23 (aA) | |
| Orchard 4 | 35.76 ± 4.64 (cAB) | 18.28 ± 0.91 (bB) | 23.42 ± 0.34 (bB) | 23 ± 2.33 (bB) | 3.9 ± 0.47 (aA) | 2.72 ± 0.12 (aA) | 2.05 ± 0.11 (aA) | |
| Orchard 5 | 17.75 ± 3.28 (bA) | 8.61 ± 0.61 (aA) | 5.57 ± 1.18 (aA) | 7.21 ± 2.24 (aA) | 6.16 ± 0.18 (aB) | 5.33 ± 0.57 (aB) | 2.42 ± 0.05 (aA) |
| Treatments | 0 d | 15 d | 30 d | 45 d | 60 d | 75 d | 90 d | |
|---|---|---|---|---|---|---|---|---|
| POL | Orchard 1 (OF) | 110 ± 15 (bcD) | 151.2 ± 3.56 (dD) | 178.22 ± 2 (eE) | 188.21 ± 1.44 (eD) | 142.43 ± 3.68 (cdC) | 125.14 ± 2.9 (abB) | 91.56 ± 1.18 (aB) |
| Orchard 2 | 115.13 ± 2.5 (bC) | 122.96 ± 1.05 (bB) | 124.07 ± 3.22 (bC) | 125.3 ± 0.86 (bB) | 88.27 ± 6.33 (aA) | 91.56 ± 3.16 (aA) | 80.06 ± 3.11 (aA) | |
| Orchard 3 | 95.57 ± 3.12 (aB) | 99.82 ± 3.77 (aA) | 107.20 ± 3.39 (abB) | 117.31 ± 3.03 (bB) | 122.18 ± 0.58 (bcB) | 133.91 ± 3.37 (bcB) | 121.22 ± 2.49 (cB) | |
| Orchard 4 | 78.51 ± 0.53 (aA) | 123.21 ± 2.94 (cB) | 138.72 ± 1.42 (deD) | 164.65 ± 2.04 (fC) | 110.53 ± 2.18 (bB) | 133.87 ± 2.29 (cdB) | 147.30 ± 4.59 (efC) | |
| Orchard 5 | 87.01 ± 2.58 (abcAB) | 100.53 ± 1.84 (dA) | 79.44 ± 1.35 (aA) | 82.01 ± 1.88 (abA) | 90.48 ± 2.49 (bcdA) | 94.40 ± 2.97 (cdA) | 92.95 ± 1.93 (cdA) | |
| FLAV | Orchard 1 (OF) | 23.76 ± 0.81 (bB) | 19.05 ± 0.5 (aC) | 17.88 ± 0.64 (aC) | 22.76 ± 0.56 (bC) | 22.89 ± 0.45 (bD) | 19.65 ± 0.35 (aB) | 19.79 ± 0.25 (aD) |
| Orchard 2 | 23.88 ± 0.83 (dB) | 14.37 ± 0.49 (bB) | 15.33 ± 0.42 (bcBC) | 17.70 ± 0.7 (cB) | 14.50 ± 0.26 (bC) | 10.24 ± 0.22 (aA) | 13.15 ± 0.32 (bC) | |
| Orchard 3 | 23.61 ± 0.51 (eB) | 11.36 ± 0.66 (cA) | 14.11 ± 0.54 (dB) | 15.42 ± 0.55 (dB) | 7.42 ± 0.04 (abA) | 8.63 ± 0.52 (bA) | 5.77 ± 0.51 (aA) | |
| Orchard 4 | 16.23 ± 0.81 (dA) | 11.83 ± 0.49 (bcA) | 13.18 ± 0.82 (cB) | 17.69 ± 0.53 (dB) | 6.23 ± 0.27 (aA) | 9.3 ± 0.27 (bA) | 9.21 ± 0.26 (bB) | |
| Orchard 5 | 12.86 ± 0.54 (bA) | 11.99 ± 0.2 (bA) | 9.72 ± 0.42 (aA) | 10.03 ± 0.11 (aA) | 11.48 ± 0.23 (abB) | 10.12 ± 0.48 (aA) | 9.68 ± 0.44 (aB) | |
| AA | Orchard 1 (OF) | 70.40 ± 0.7 (dC) | 66.58 ± 1.65 (bcdC) | 68.01 ± 1.87 (dB) | 65.56 ± 2.87 (cdC) | 58.44 ± 3.56 (abcB) | 57.32 ± 2.08 (abC) | 53.34 ± 1.72 (aC) |
| Orchard 2 | 58.9 ± 2.52 (cB) | 46.01 ± 3.03 (abAB) | 43.14 ± 2.44 (aA) | 48.73 ± 1.19 (abcAB) | 53.53 ± 1.57 (bcAB) | 54.26 ± 2.24 (bcBC) | 51.72 ± 0.94 (abcBC) | |
| Orchard 3 | 38.03 ± 1.77 (aA) | 43.79 ± 2.51 (aA) | 42.80 ± 1.48 (aA) | 41.09 ± 1.89 (aA) | 45.71 ± 3.9 (aAB) | 46.44 ± 1.03 (aAB) | 44.74 ± 1.92 (aAB) | |
| Orchard 4 | 45.24 ± 3.17 (aA) | 53.26 ± 0.6 (aB) | 48 ± 3.91 (aA) | 52.16 ± 2.58 (aB) | 43.76 ± 2.25 (aA) | 44.79 ± 0.7 (aA) | 41.93 ± 1.81 (aA) | |
| Orchard 5 | 42.28 ± 2.87 | 47.49 ± 1.37 (abAB) | 51.23 ± 2.64 (abA) | 55.88 ± 2.45 (bB) | 48.57 ± 2.15 (abAB) | 55.67 ± 1.77 (bC) | 53.42 ± 1.20 (bC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, C.; Forniti, R.; Botondi, R. Post-Harvest Quality Evaluation of “Soreli” Kiwifruit at Two Ripening °Brix Values from Vineyards of Different Age Under Hail Nets. Foods 2022, 11, 431. https://doi.org/10.3390/foods11030431
Grasso C, Forniti R, Botondi R. Post-Harvest Quality Evaluation of “Soreli” Kiwifruit at Two Ripening °Brix Values from Vineyards of Different Age Under Hail Nets. Foods. 2022; 11(3):431. https://doi.org/10.3390/foods11030431
Chicago/Turabian StyleGrasso, Claudia, Roberto Forniti, and Rinaldo Botondi. 2022. "Post-Harvest Quality Evaluation of “Soreli” Kiwifruit at Two Ripening °Brix Values from Vineyards of Different Age Under Hail Nets" Foods 11, no. 3: 431. https://doi.org/10.3390/foods11030431
APA StyleGrasso, C., Forniti, R., & Botondi, R. (2022). Post-Harvest Quality Evaluation of “Soreli” Kiwifruit at Two Ripening °Brix Values from Vineyards of Different Age Under Hail Nets. Foods, 11(3), 431. https://doi.org/10.3390/foods11030431