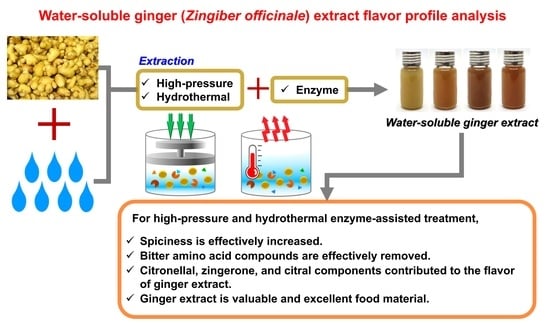
Effects of High-Pressure, Hydrothermal, and Enzyme-Assisted Treatment on the Taste and Flavor Profile of Water-Soluble Ginger (Zingiber officinale) Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Enzymes and Chemicals
2.1.2. Plant Materials and Sample Preparation
2.2. Extraction
2.2.1. Squeezed Raw Ginger Juice and Tea
2.2.2. Hydrothermal Extraction
2.2.3. High-Pressure Extraction
2.3. Extraction Yield and Sugar Content Measurement
2.4. Analysis of Taste Compounds
2.4.1. Gingerols and Shogaols Analysis
2.4.2. Free Amino Acid (AA) Analysis
2.5. Analysis of Volatile Compounds
2.5.1. Electronic Nose Analysis
2.5.2. SBSE-TD/GC-MS/MS Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield and Sugar Contents
3.2. Taste Compounds Properties
3.2.1. Spiciness: Gingerol and Shogaol
3.2.2. Bitterness, Umami, Salty, Sourness and Sweetness: Free Amino Acids
3.3. Volatile Compounds Properties
3.3.1. Radar Charts and PCA by E-nose
3.3.2. Comprehensive Analysis of SBSE-TD/GC-MS/MS Volatile Compounds
3.3.3. Comparison of Total Volatile Compounds by Extraction Method
3.3.4. Different Extraction Methods Affected Ginger Extract Flavor Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heasman, M. Sugar and the modern food system. Br. Food J. 1989, 91, 9–16. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Unni, L.E.; Chauhan, O.P.; Raju, P.S. Quality changes in high pressure processed ginger paste under refrigerated storage. Food Biosci. 2015, 10, 18–25. [Google Scholar] [CrossRef]
- Mañas, P.; Pagán, R. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 2005, 98, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPME-GC–MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Tubtimdee, C.; Shotipruk, A. Extraction of phenolics from Terminalia chebula Retz with water–ethanol and water–propylene glycol and sugaring-out concentration of extracts. Sep. Purify. Technol. 2011, 77, 339–346. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 6. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Analyt. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Ghandahari Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci. Nutr. 2019, 7, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Azian, M.N.; Kamal, A.M.; Azlina, M.N. Changes of cell structure in ginger during processing. J. Food Eng. 2004, 62, 359–364. [Google Scholar] [CrossRef]
- Kim, J.S.; Koh, M.S.; Kim, Y.H.; Kim, M.K.; Hong, J.S. Volatile flavor components of Korean ginger (Zingiber officinale Roscoe). Korean J. Food Sci. Technol. 1991, 23, 141–149. Available online: https://www.koreascience.or.kr/article/JAKO199103041968952.pdf (accessed on 17 January 2022).
- Yonei, Y.; Ōhinata, H.; Yoshida, R.; Shimizu, Y.; Yokoyama, C. Extraction of ginger flavor with liquid or supercritical carbon dioxide. J. Supercrit. Fluids. 1995, 8, 156–161. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile essential oils, aromas and flavours: Degradation pathways, effect of thermal processing and alteration of sensory quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Z.H.; Guo, X.F.; Hu, X.S.; Wu, J.H. Geographical origin identification of propolis using GC–MS and electronic nose combined with principal component analysis. Food Res. Int. 2013, 51, 813–822. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fuentes, A.; Fernández-Segovia, I.; Vivancos, J.L.; Rizo, A.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Evaluation of sea bream (Sparus aurata) shelf life using an optoelectronic nose. Food Chem. 2013, 138, 1374–1380. [Google Scholar] [CrossRef]
- Nam, D.G.; Kim, M.; Im, P.; Kim, S.B.; Choe, J.S.; Choi, A.J. Solubilization of polysaccharide and functional components by high-pressure enzyme treatment from ginger (Zingiber officinale Rosc.). Food Eng. Prog. 2018, 22, 173–185. [Google Scholar] [CrossRef]
- Nam, D.G.; Kim, M.; Im, P.; Choe, J.S.; Choi, A.J. Solubilization of polysaccharides and functional components of ginger (Zingiber officinale Rosc.) using ethanol and enzyme. Korean J. Food Preserv. 2019, 26, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Henderson, J.H.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate and Reproducible HPLC Analysis of Amino Acids 10 Amino Acid Analysis Using Zorbax Eclipse AAA Columns and the Agilent 1100 HPLC; Part No. 5980-1193E; Agilent Technologies: Santa Clara, CA, USA, 2000; Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2000&pages=10&author=J+H+Henderson&author=R+D+Ricker&author=B+A+Bidlingmeyer&author=C+Woodward&title=+Rapid%2C+accurate+and+reproducible+HPLC+analysis+of+amino+acids+ (accessed on 17 January 2022).
- Wiśniewska, P.; Śliwińska, M.; Namieśnik, J.; Wardencki, W.; Dymerski, T. The verification of the usefulness of electronic nose based on ultra-fast gas chromatography and four different chemometric methods for rapid analysis of spirit beverages. J. Anal. Methods Chem. 2016, 2016, 8763436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seethapathy, S.; Gorecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta. 2012, 750, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Marsol-Vall, A.; Balcells, M.; Eras, J.; Canela-Garayoa, R. Development of a SBSE-TD method coupled to GC–MS and chemometrics for the differentiation of variety and processing conditions in peach juices. Food Chem. 2018, 239, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, T.; He, L.; Xie, J.; Chen, C.; Tian, H. Characterization of aroma compounds in bayberry juice by sensory evaluation and gas chromatography–mass spectrometry. J. Food Meas. Charact. 2020, 14, 505–513. [Google Scholar] [CrossRef]
- Vasala, P.A.; Ginger Peter, K.V. Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 3, pp. 319–335. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kramer, T.; Kulow, K. The effect of spicy gustatory sensations on variety-seeking. Psychol Mark. 2017, 34, 786–794. [Google Scholar] [CrossRef]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef]
- Selig, M.J.; Celli, G.B.; Tan, C.; La, E.; Mills, E.; Webley, A.D.; Padilla-Zakour, O.I.; Abbaspourrad, A. High pressure processing of beet extract complexed with anionic polysaccharides enhances red color thermal stability at low pH. Food Hydrocoll. 2018, 80, 292–297. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Running, C.A.; Craig, B.A.; Mattes, R.D. Oleogustus: The unique taste of fat. Chem. Sens. 2015, 40, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, O.B.; Akomolafe, S.F.; Akinyemi, F.T. Food value of two varieties of ginger (Zingiber officinale) commonly consumed in Nigeria. Int. Sch. Res. Not. 2013, 2013, 359727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shomer, I.; Yefremov, T.; Merin, U. Involvement of proteins in cloud instability of Shamouti orange [Citrus sinensis (L.) Osbeck] juice. J. Agric. Food Chem. 1999, 47, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Ninga, K.A.; Desobgo, Z.S.C.; De, S.; Nso, E.J. Pectinase hydrolysis of guava pulp: Effect on the physicochemical characteristics of its juice. Heliyon 2021, 7, e08141. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Elmore, J.S.; Mottram, D.S. Comparison of the aroma characteristics of acid-hydrolyzed and enzyme-hydrolyzed vegetable proteins produced from soy. J. Agric. Food Chem. 1998, 46, 5225–5231. [Google Scholar] [CrossRef]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Ninomiya, T.; Katsuya, N. Contribution of peptides and amino acids to the taste of foods. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Kemp, S.E.; Birch, G.G. An intensity/time study of the taste of amino acids. Chem. Sens. 1992, 17, 151–168. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of amino acid taste and appetite. Adv. Nutr. 2016, 7, 806S–822S. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.Y.; Kwak, H.S.; Kim, M.J.; Kim, Y.; Kim, K.O.; Kim, S.S. Comparison of a descriptive analysis and instrumental measurements (electronic nose and electronic tongue) for the sensory profiling of Korean fermented soybean paste (doenjang). J. Sens. Stud. 2017, 32, e12282. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef]
- Cheng, A.X.; Lou, Y.G.; Mao, Y.B.; Lu, S.; Wang, L.J.; Chen, X.Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The structure and function of major plant metabolite modifications. Mol. Plant. 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Guo, D.; Kang, K.; Wang, P.; Li, M.; Huang, X. Transcriptome profiling of spike provides expression features of genes related to terpene biosynthesis in lavender. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paramita, V.; Yulianto, M.E.; Hartati, I.; Bahrudin, U.; Amalia, R.; Kusumo, P.; Alam Syah, A.N.; Arimukti, T.K. Pressurized Hot Water Extraction of Zingiber officinale Fresh Rhizome. Adv. Sci. Lett. 2018, 24, 9856–9858. [Google Scholar] [CrossRef]
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Yousif, A.N.; Scaman, C.H.; Durance, T.D.; Girard, B. Flavor volatiles and physical properties of vacuum-microwave-and air-dried sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 1999, 47, 4777–4781. [Google Scholar] [CrossRef]
- Chen, C.C.; Ho, C.T. Volatile compounds in ginger oil generated by thermal treatment. ACS Symp. Ser. 1989, 409, 366–375. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Chuang, C.H.; Chen, H.C.; Wan, C.J.; Chen, T.L.; Lin, L.Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Bartley, J.P.; Jacobs, A.L. Effects of drying on flavour compounds in Australian-grown ginger (Zingiber officinale). J. Sci. Food Agric. 2000, 80, 209–215. [Google Scholar] [CrossRef]
- An, K.; Tang, D.; Wu, J.; Fu, M.; Wen, J.; Xiao, G.; Xu, Y. Comparison of pulsed vacuum and ultrasound osmotic dehydration on drying of Chinese ginger (Zingiber officinale Roscoe): Drying characteristics, antioxidant capacity, and volatile profiles. Food Sci. Nutr. 2019, 7, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Lubinska-Szczygeł, M.; Pudlak, D.; Dymerski, T.; Namieśnik, J. Rapid assessment of the authenticity of limequat fruit using the electronic nose and gas chromatography coupled with mass spectrometry. Monatsh. Chem. 2018, 149, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarichian, A.; Chayjan, R.A.; Ahmadi, E.; Mohtasebi, S.S. Assessment the influence of different drying methods and pre-storage periods on garlic (Allium sativum L.) aroma using electronic nose. Food Bioprod. Process. 2021, 127, 198–211. [Google Scholar] [CrossRef]






| Pre-Treatment Method | Symbol | Yield (%) | Bx° |
|---|---|---|---|
| Squeezed juice/raw | GJ | 1.11 ± 0.01 f | 1.10 ± 0.00 e |
| Hot-water/leached/powder | GT | 16.26 ± 0.18 e | 1.83 ± 0.06 b |
| Hydrothermal/powder | HW | 22.66 ± 0.23 d | 1.20 ±0.00 d |
| Hydrothermal/enzyme/powder | HWE | 55.40 ± 1.18 b | 2.57 ± 0.06 a |
| High-pressure/powder | HP | 24.46 ± 0.19 c | 1.40 ± 0.00 c |
| High-pressure/enzyme/powder | HPE | 67.60 ± 0.48 a | 2.53 ± 0.06 a |
| F-value | 6575.07 *** | 767.53 *** |
| GJ | GT | HW | HWE | HP | HPE | F-Value | |
|---|---|---|---|---|---|---|---|
| 6G (mg/g) | 0.81 ± 0.00 f | 1.27 ± 0.02 e | 2.28 ± 0.03 c | 7.03 ± 0.01 a | 2.18 ± 0.03 d | 6.30 ± 0.01 b | 50,470.25 *** |
| 8G (mg/g) | 0.17 ± 0.00 f | 0.22 ± 0.00 e | 0.50 ± 0.00 c | 1.67 ± 0.00 a | 0.47 ± 0.00 d | 1.53 ± 0.00 b | 79,353.60 *** |
| 10G (mg/g) | 0.48 ± 0.00 f | 0.73 ± 0.01 e | 1.37 ± 0.01 c | 4.08 ± 0.01 a | 1.31 ± 0.01 d | 3.60 ± 0.03 b | 52,907.18 *** |
| 6S (mg/g) | 0.29 ± 0.00 f | 0.44 ± 0.00 e | 0.86 ± 0.00 c | 2.52 ± 0.01 b | 0.83 ± 0.00 d | 2.62 ± 0.01 a | 116,407.04 *** |
| 8S (mg/g) | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.04 ± 0.00 c | 0.39 ± 0.00 b | 0.04 ± 0.00 c | 0.41 ± 0.00 a | 10,460.00 *** |
| 10S (mg/g) | 0.15 ± 0.01 c | 0.22 ± 0.02 c | 0.48 ± 0.03 b | 1.44 ± 0.09 a | 0.46 ± 0.03 b | 1.44 ± 0.01 a | 574.91 *** |
| Intensity | Taste | GJ | GT | HW | HWE | HP | HPE | F-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Essential amino acid (mg/100 g) | Val | Slight | Bitter | 14.38 ± 0.51 c | 86.39 ± 3.88 a | 81.31 ± 1.46 b | 6.74 ± 1.58 d | 76.92 ± 4.59 b | 2.14 ± 0.27 d | 725.61 *** |
| Leu | Extreme | Bitter | 7.33 ± 0.27 d | 41.85 ± 1.61 c | 50.03 ± 0.98 a | 2.45 ± 0.22 e | 47.02 ± 2.41 b | 1.56 ± 0.02 e | 1049.34 *** | |
| Iso | Moderate | Bitter | 6.31 ± 0.23 d | 38.31 ± 1.56 c | 44.04 ± 1.07 a | 1.18 ± 0.21 e | 41.15 ± 2.32 b | 0.70 ± 0.02 e | 893.61 *** | |
| Lys | Slight | Bitter, Salty | 3.37 ± 0.19 e | 53.76 ± 1.04 a | 47.89 ± 0.49 b | 5.87 ± 0.29 d | 44.04 ± 2.24 c | 2.52 ± 0.12 e | 1689.51 *** | |
| Thr | Slight | Sweet | 11.32 ± 0.35 d | 50.25 ± 2.22 a | 46.91 ± 0.90 b | 0.99 ± 0.02 e | 43.68 ± 2.78 c | 1.14 ± 0.05 e | 739.05 *** | |
| Phe | Extreme | Bitter | 4.57 ± 0.16 c | 28.48 ± 1.07 b | 31.16 ± 0.61 a | 1.88 ± 0.17 d | 29.51 ± 1.59 b | 2.19 ± 0.02 d | 957.72 *** | |
| Met | Extreme | Bitter | 5.32 ± 0.21 d | 7.18 ± 0.08 b | 7.31 ± 0.13 ab | 6.43 ± 0.22 c | 7.71 ± 0.46 a | 0.40 ± 0.01 e | 411.70 *** | |
| His | Extreme | Bitter | 11.28 ± 0.34 c | 12.39 ± 0.36 c | 19.95 ± 0.43 a | 0.17 ± 0.02 d | 18.04 ± 1.49 b | 0.36 ± 0.04 d | 487.65 *** | |
| Tyr | Extreme | Bitter | 7.82 ± 0.28 c | 71.61 ± 3.73 b | 86.40 ± 1.45 a | 1.53 ± 0.14 d | 81.94 ± 4.97 a | 2.32 ± 0.02 d | 777.03 *** | |
| Non-essential amino acid (mg/100 g) | Gln | Slight | Sweet, Salty, Bitter | 0.22 ± 0.01 | ND | 2.19 ± 0.10 | ND | 0.77 ± 0.17 | ND | NS |
| Asn | - | Tasteless | ND | ND | 120.94 ± 2.60 | ND | 108.90 ± 3.79 | ND | NS | |
| Glu | Extreme | Umami, Sweet | 81.21 ± 2.82 c | 154.42 ± 4.00 b | 239.90 ± 5.43 a | 7.62 ± 0.30 d | 230.30 ± 16.38 a | 7.63 ± 0.06 d | 609.76 *** | |
| Arg | Moderate | Bitter | 0.13 ± 0.02 e | 3.49 ± 1.94 cd | 77.63 ± 1.30 a | 4.83 ± 0.15 c | 69.70 ± 2.63 b | 0.94 ± 0.02 de | 1988.54 *** | |
| Ala | Moderate | Umami, Sweet | 45.59 ± 1.57 b | 181.41 ± 7.18 a | 189.35 ± 3.50 a | 3.76 ± 0.12 c | 180.19 ± 11.48 a | 3.85 ± 0.09 c | 772.78 *** | |
| Pro | Moderate | Bitter, Sweet | 7.29 ± 0.48 c | 2.02 ± 0.58 d | 70.56 ± 1.64 a | 0.66 ± 0.03 de | 64.03 ± 0.57 b | 0.56 ± 0.01 e | 5646.38 *** | |
| Asp | Extreme | Umami | 93.46 ± 3.16 c | 24.27 ± 2.34 d | 245.80 ± 4.19 a | 3.66 ± 0.19 e | 230.38 ± 12.73 b | 3.13 ± 0.03 e | 1156.88 *** | |
| Ser | Moderate | Sweet | 49.24 ± 1.67 c | 6.82 ± 0.34 d | 119.63 ± 3.04 a | 1.19 ± 0.09 e | 102.99 ± 3.25 b | 1.38 ± 0.05 e | 2251.65 *** | |
| Gly | Moderate | Sweet | 8.00 ± 0.32 c | 77.48 ± 3.55 a | 57.46 ± 1.59 b | 0.62 ± 0.05 d | 54.13 ± 3.06 b | 1.32 ± 0.03 d | 836.63 *** | |
| Try | Slight | Bitter | 8.46 ± 0.13 b | 10.70 ± 4.61 b | 12.11 ± 1.09 ab | 14.98 ± 0.07 a | 10.80 ± 1.90 b | 2.38 ± 0.13 d | 12.53 *** | |
| Other amino acid (mg/100 g) | Orn | Slight | Sour | 5.18 ± 0.11 de | 43.19 ± 1.03 a | 29.13 ± 1.75 b | 6.58 ± 0.15 d | 26.15 ± 1.94 c | 3.25 ± 0.14 e | 605.70 *** |
| Cit | Extreme | Sour | 26.91 ± 0.92 b | 33.98 ± 1.71 a | 11.08 ± 0.43 d | 18.87 ± 0.16 c | 10.68 ± 0.73 d | 1.61 ± 0.10 e | 560.48 *** | |
| GABA | - | Tasteless | 6.47 ± 0.21 c | 56.97 ± 1.85 a | 56.42 ± 1.17 ab | 1.78 ± 0.09 d | 53.53 ± 3.34 b | 1.18 ± 0.02 d | 935.86 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, D.-G.; Kim, M.; Choe, J.-S.; Choi, A.-j. Effects of High-Pressure, Hydrothermal, and Enzyme-Assisted Treatment on the Taste and Flavor Profile of Water-Soluble Ginger (Zingiber officinale) Extract. Foods 2022, 11, 508. https://doi.org/10.3390/foods11040508
Nam D-G, Kim M, Choe J-S, Choi A-j. Effects of High-Pressure, Hydrothermal, and Enzyme-Assisted Treatment on the Taste and Flavor Profile of Water-Soluble Ginger (Zingiber officinale) Extract. Foods. 2022; 11(4):508. https://doi.org/10.3390/foods11040508
Chicago/Turabian StyleNam, Dong-Geon, Mina Kim, Jeong-Sook Choe, and Ae-jin Choi. 2022. "Effects of High-Pressure, Hydrothermal, and Enzyme-Assisted Treatment on the Taste and Flavor Profile of Water-Soluble Ginger (Zingiber officinale) Extract" Foods 11, no. 4: 508. https://doi.org/10.3390/foods11040508
APA StyleNam, D. -G., Kim, M., Choe, J. -S., & Choi, A. -j. (2022). Effects of High-Pressure, Hydrothermal, and Enzyme-Assisted Treatment on the Taste and Flavor Profile of Water-Soluble Ginger (Zingiber officinale) Extract. Foods, 11(4), 508. https://doi.org/10.3390/foods11040508