Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability
Abstract
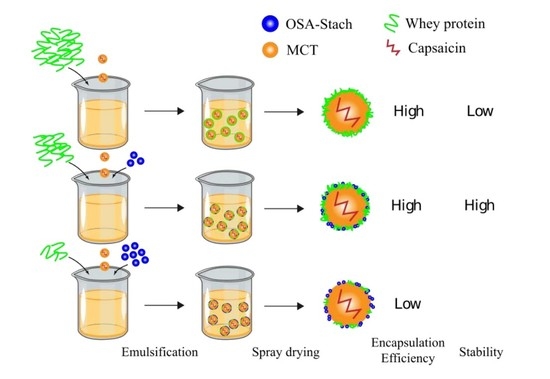
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Modification by OSA
2.3. Preparation of Capsaicin Emulsion and Microcapsules
2.4. Light Microscopy (LM)
2.5. Fluorescence Microscopy (AFM)
2.6. Rheological Properties
2.7. Characterization of Microcapsules
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Fourier Transform-Infrared Spectroscopy (FT-IR)
2.7.3. X-ray Diffraction (XRD) Pattern
2.7.4. Yield of Encapsulation
2.7.5. Encapsulation Efficiency of Microcapsules
- (1).
- The standard curve of capsaicin
- (2).
- The total capsaicin content measurement
- (3).
- The unencapsulated capsaicin measurement
2.7.6. The Moisture Content
2.7.7. Solubility Measurement
2.7.8. Wettability of Microcapsules
2.7.9. Color Measurement
2.7.10. Thermal Properties Measurement
2.7.11. Particle Size Distribution
2.8. Stability of the Capsaicin Microcapsules
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characteristics of Capsaicin Emulsion
3.2. Rheological Properties of Capsaicin Emulsion
3.3. SEM and Appearance Analysis
3.4. FT-IR Spectroscopy Analysis
3.5. X-ray Diffraction Patterns
3.6. Yield, Encapsulation Efficiency, Moisture, Wettability, and Solubility
3.7. Color Analysis
3.8. Thermal Properties
3.9. Stability of the Capsaicin Microcapsules
3.10. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, J.; Zhang, S.; Liu, X.; Xiao, C. Fabrication of capsaicin emulsions: Improving the stability of the system and relieving the irritation to the gastrointestinal tract of rats. J. Sci. Food Agric. 2020, 100, 129–138. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, H.; Zhou, C.; Lv, F.; Bie, X.; Lu, Z. Study on the spray-drying encapsulation of lutein in the porous starch and gelatin mixture. Eur. Food Res. Technol. 2012, 234, 157–163. [Google Scholar] [CrossRef]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Cartagena, J.C.; Alzate, L.M.; Londoño-Londoño, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Umaña, M.; Turchiuli, C.; Rosselló, C.; Simal, S. Addition of a mushroom by-product in oil-in-water emulsions for the microencapsulation of sunflower oil by spray drying. Food Chem. 2021, 343, 128429. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A. Interactions and functionality of milk proteins in food emulsions. Milk Proteins 2014, 12, 359–386. [Google Scholar] [CrossRef]
- Jimenez, M.; García, H.S.; Beristain, C.I. Spray-drying microencapsulation and oxidative stability of conjugated linoleic acid. Eur. Food Res. Technol. 2004, 219, 588–592. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Romero-Hernandez, H.A.; Sánchez-Rivera, M.M.; Alvarez-Ramirez, J.; Yee-Madeira, H.; Yañez-Fernandez, J.; Bello-Pérez, L.A. Avocado oil encapsulation with OSA-esterified taro starch as wall material: Physicochemical and morphology characteristics. LWT-Food Sci. Technol. 2021, 138, 110629. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Korma, S.A.; Khan, I.M.; Mohsin, A.; Manzoor, M.F.; Ashraf, W.; Mushtaq, B.S.; Zainab, S.; et al. Spray dried nanoemulsions loaded with curcumin, resveratrol, and borage seed oil: The role of two different modified starches as encapsulating materials. Int. J. Biol. Macromol. 2021, 186, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2020, 101, 105503. [Google Scholar] [CrossRef]
- He, J.; Liu, J.; Zhang, G. Slowly digestible waxy maize starch prepared by octenyl succinic anhydride esterification and heat-moisture treatment: Glycemic response and mechanism. Biomacromolecules 2008, 9, 175–184. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, F.; Duan, X.; Xiao, C.; Liu, X. Physicochemical Properties of Lutein-Loaded Microcapsules and Their Uptake via Caco-2 Monolayers. Molecules 2018, 23, 1805. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Association of Analytical Chemists: Washington, DC, USA, 1997. [Google Scholar]
- Wang, R.; Wan, J.; Liu, C.; Xia, X.; Ding, Y. Pasting, thermal, and rheological properties of rice starch partially replaced by inulin with different degrees of polymerization. Food Hydrocoll. 2019, 92, 228–232. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, D.; Özkan, N.; Chen, X.D.; Mao, Z. Effect of flaxseed gum addition on rheological properties of native maize starch. J. Food Eng. 2008, 89, 87–92. [Google Scholar] [CrossRef]
- Rosenberg, M.; Young, S.L. Whey proteins as microencapsulating agents. Microencapsulation of anhydrous milkfat-structure evaluation. Food Struct. 1993, 12, 31–41. [Google Scholar]
- Niazi, M.B.K.; Broekhuis, A.A. Production of amorphous starch powders by solution spray drying. J. Appl. Polym. Sci. 2012, 126, E143–E153. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujitaa, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using beta-cyclodextrin/gumarabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef]
- Fernandes, R.V.B.; Borges, S.V.; Botrel, D.A. Gum Arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.; Bhandari, B.R.; Howes, T. Optimization of co-current spray drying process of sugar-rich foods. Part I-Moisture and glass transition temperature profile during drying. J. Food Eng. 2005, 71, 55–65. [Google Scholar] [CrossRef]
- Corrigan, O.I. Thermal analysis of spray dried products. Thermochim. Acta 1995, 248, 245–258. [Google Scholar] [CrossRef]
- Botrel, D.A.; Fernandes, R.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Liu, C.H.; Lai, K.Y.; Wu, W.C.; Chen, Y.J.; Lee, W.S.; Hsu, C.Y. In vitro scleral lutein distribution by cyclodextrin containing nanoemulsions. Chem. Pharm. Bull. 2015, 63, 59–67. [Google Scholar] [CrossRef]
- Li, J.; Shin, G.H.; Lee, I.W.; Chen, X.; Park, H.J. Soluble starch formulated nanocomposite increases water solubility and stability of curcumin. Food Hydrocoll. 2016, 56, 41–49. [Google Scholar] [CrossRef]






| Temperatures | WP:OS | τ0 (Pa) | K (Pa·s−1) | n | R2 |
|---|---|---|---|---|---|
| 25 °C | 10:0 | 0.0062 ± 0.0001 ab | 0.0034 ± 0.0001 f | 0.8667 ± 0.0053 c | 0.9965 |
| 9:1 | 0.0021 ± 0.0003 b | 0.0084 ± 0.0000 e | 0.9518 ± 0.0006 a | 0.9998 | |
| 7:3 | −0.0081 ± 0.0014 c | 0.0294 ± 0.0027 c | 0.8320 ± 0.0202 d | 0.9968 | |
| 5:5 | 0.0126 ± 0.0008 a | 0.0163 ± 0.0001 d | 0.9077 ± 0.0021 b | 0.9909 | |
| 3:7 | 0.0071 ± 0.0008 ab | 0.0289 ± 0.0002 c | 0.8548 ± 0.0019 cd | 0.9989 | |
| 1:9 | 0.0025 ± 0.0000 b | 0.1276 ± 0.0001 b | 0.7039 ± 0.0010 f | 0.9987 | |
| 0:10 | −0.0306 ± 0.0048 d | 0.1771 ± 0.0006 a | 0.7559 ± 0.0005 e | 0.9991 | |
| 50 °C | 10:0 | 0.0018 ± 0.0006 ab | 0.0012 ± 0.0001 f | 0.9852 ± 0.0188 b | 0.9930 |
| 9:1 | 0.0036 ± 0.0000 a | 0.0018 ± 0.0000 f | 1.0531 ± 0.0016 a | 0.9981 | |
| 7:3 | −0.0073 ± 0.0012 b | 0.0384 ± 0.0028 c | 0.6753 ± 0.0178 f | 0.9930 | |
| 5:5 | 0.0007 ± 0.0005 ab | 0.0097 ± 0.0007 e | 0.8809 ± 0.0163 c | 0.9978 | |
| 3:7 | −0.0023 ± 0.0002 ab | 0.0172 ± 0.0000 d | 0.8650 ± 0.0007 c | 0.9977 | |
| 1:9 | −0.0336 ± 0.0066 d | 0.0903 ± 0.0024 b | 0.7485 ± 0.0055 e | 0.9978 | |
| 0:10 | −0.0230 ± 0.0006 c | 0.1026 ± 0.0014 a | 0.8168 ± 0.0032 d | 0.9993 |
| WP:OS | Yield (%) | Encapsulation Efficiency (%) | Water (%) | Wettability (s) | Solubility (%) |
|---|---|---|---|---|---|
| 10:0 | 68.18 ± 0.51 a | 94.57 ± 0.64 a | 0.33 ± 0.05 c | 158.87 ± 3.31 d | 96.57 ± 0.14 a |
| 9:1 | 64.26 ± 0.05 b | 93.51 ± 0.79 a | 0.48 ± 0.02 c | 187.31 ± 1.60 b | 92.35 ± 0.05 b |
| 7:3 | 55.51 ± 0.63 c | 90.43 ± 0.89 a | 1.67 ± 0.56 bc | 172.86 ± 1.68 c | 85.77 ± 1.14 c |
| 5:5 | 48.39 ± 0.34 d | 80.85 ± 0.39 b | 1.30 ± 0.48 c | 168.33 ± 3.54 cd | 80.06 ± 0.62 d |
| 3:7 | 38.47 ± 0.49 e | 74.37 ± 2.05 c | 5.35 ± 0.14 a | 195.63 ± 0.98 b | 77.50 ± 0.01 e |
| 1:9 | 9.32 ± 0.50 f | 49.91 ± 2.58 d | 3.00 ± 0.46 b | 232.63 ± 2.68 a | 74.99 ± 0.62 f |
| WP:OS | The Color Parameters | d (4, 3) (μm) | d (3, 2) (μm) | Particle Size Distributions (μm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| L* (%) | a* (%) | b* (%) | ΔE | D (10) | D (50) | D (90) | |||
| 10:0 | 93.78 ± 0.39 a | 2.13 ± 0.16 d | 15.96 ± 0.81 d | 0.59 ± 0.08 d | 3.22 ± 0.21 c | 1.49 ± 0.04 e | 0.76 ± 0.01 f | 1.78 ± 0.06 c | 8.13 ± 2.07 c |
| 9:1 | 93.41 ± 0.22 a | 2.52 ± 0.09 d | 17.27 ± 0.57 d | 0.98 ± 0.56 d | 24.53 ± 0.35 b | 3.95 ± 0.07 d | 1.24 ± 0.03 e | 23.87 ± 0.58 a | 51.57 ± 0.72 a |
| 7:3 | 93.05 ± 0.60 a | 2.83 ± 0.33 d | 18.27 ± 1.45 d | 2.11 ± 1.53 d | 24.60 ± 0.36 b | 4.79 ± 0.20 c | 1.63 ± 0.04 d | 23.53 ± 0.61 a | 49.83 ± 0.23 ab |
| 5:5 | 89.85 ± 0.23 b | 8.00 ± 0.11 c | 24.44 ± 0.61 c | 10.63 ± 0.57 c | 24.20 ± 0.36 b | 5.82 ± 0.05 b | 2.35 ± 0.03 c | 22.37 ± 0.50 b | 48.17 ± 0.47 b |
| 3:7 | 87.71 ± 0.10 c | 10.76 ± 0.12 b | 29.07 ± 0.12 b | 16.42 ± 0.18 b | 24.70 ± 0.17 b | 7.94 ± 0.33 a | 3.67 ± 0.06 b | 22.90 ± 0.17 ab | 47.40 ± 0.35 b |
| 1:9 | 86.60 ± 0.43 d | 12.48 ± 0.50 a | 32.84 ± 0.95 a | 20.65 ± 1.14 a | 26.03 ± 0.23 a | 8.16 ± 0.25 a | 6.72 ± 0.06 a | 23.97 ± 0.21 a | 48.40 ± 0.61 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zheng, L.; Liang, S.; Lu, Y.; Zheng, J.; Zhang, G.; Li, W.; Jiang, H. Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods 2022, 11, 612. https://doi.org/10.3390/foods11040612
Zhang B, Zheng L, Liang S, Lu Y, Zheng J, Zhang G, Li W, Jiang H. Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods. 2022; 11(4):612. https://doi.org/10.3390/foods11040612
Chicago/Turabian StyleZhang, Bo, Luyao Zheng, Siyuan Liang, Yifan Lu, Jianmei Zheng, Guoquan Zhang, Wenhao Li, and Hao Jiang. 2022. "Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability" Foods 11, no. 4: 612. https://doi.org/10.3390/foods11040612
APA StyleZhang, B., Zheng, L., Liang, S., Lu, Y., Zheng, J., Zhang, G., Li, W., & Jiang, H. (2022). Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods, 11(4), 612. https://doi.org/10.3390/foods11040612