Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour
Abstract
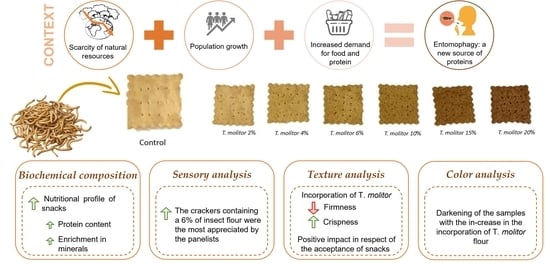
:1. Introduction
2. Materials and Methods
2.1. Production of T. molitor Flour
2.2. Crackers Preparation
2.3. Crackers Dimensions
2.4. Color Analysis
2.5. Texture Analysis
2.6. Sensory Analysis
2.7. Determination of Water Content and Water Activity (aw)
2.8. Biochemical and Mineral Composition
2.9. Total Phenolic Compounds and Antioxidant Capacity Determination
2.10. Microbiological Analysis
2.11. Statistical Analysis
3. Results
3.1. Color Analysis
3.2. Crackers Dimensions
3.3. Texture Analysis
3.4. Sensory Analysis
3.5. Total Water Content and Water Activity (aw) Determination
3.6. Biochemical and Mineral Composition Determination
3.7. Total Phenolic Compounds and Antioxidant Capacity
3.8. Determination of Microbiological Activity of 6% Crackers and Raw Flour
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects. Future Prospects for Food and Feed Security; FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Available online: http://www.fao.org/docrep/018/i3253e/i3253e00.htm (accessed on 10 January 2022).
- Much, S. Insectes Comestibles; Plume de Carotte: Toulouse, France, 2012. [Google Scholar]
- Imathiu, S. Benefits and Food Safety Concerns Associated with Consumption of Edible Insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread Enriched with Cricket Powder (Acheta Domesticus): A Technological, Microbiological and Nutritional Evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as Ingredients for Bakery Goods. A Comparison Study of H. Illucens, A. Domestica and T. Molitor Flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten Free Sourdough Bread Enriched with Cricket Flour for Protein Fortification: Antioxidant Improvement and Volatilome Characterization. Food Chem. 2020, 333, 127–410. [Google Scholar] [CrossRef] [PubMed]
- Schösler, H.; de Boer, J.; Boersema, J.J. Can We Cut out the Meat of the Dish? Constructing Consumer-Oriented Pathways towards Meat Substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 Authorising the Placing on the Market of Dried Tenebrio Molitor Larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R0882&from=EN (accessed on 10 January 2022).
- EFSA Panel. Safety of Dried Yellow Mealworm (Tenebrio Molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- Kim, S.; Keun, C.S.; Yun, E.-Y.; Ki-Byun, K.; Roh, J. Quality Characteristics of Pasta with addition of Mealworm (Tenebrio Molitor). FoodService Ind. J. 2014, 10, 55–64. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effect of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Modor Intelligence. Snack Food Market. 2021—26. Industry Share, Size, Growth—Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/snack-food-market (accessed on 8 September 2021).
- Technavio.com. Global Crackers Market 2017–2021. Technavio. Available online: https://www.technavio.com/report/global-crackers-market (accessed on 31 August 2021).
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected Species of Edible Insects as a Source of Nutrient Composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Wemans, M.D.P.C.D. Insetos Comestíveis. Avaliação Nutricional de Duas Espécies Comercializadas em Portugal. Master’s Thesis, School of Agriculture University of Lisbon (ISA), Lisbon, Portugal, 2015. Available online: https://www.repository.utl.pt/handle/10400.5/9247 (accessed on 10 January 2022).
- Zhao, X.; Vazquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes—Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [Green Version]
- Rumpold, B.A.; Schlüter, O.K. Potential and Challenges of Insects as an Innovative Source for Food and Feed Production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Williams, J.P.; Williams, J.R.; Kirabo, A.; Chester, D.; Peterson, M. Chapter 3—Nutrient Content and Health Benefits of Insects. Insects Sustain. Food Ingred. 2016, 24, 61–84. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 23, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, M. Sensory Methods of Texture and Viscosity Measurement. In Food Texture and Viscosity; Elsevier: Amsterdam, The Netherlands, 2002; pp. 259–266. ISBN 978-0-12-119062-0. [Google Scholar]
- EN ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/36385.html (accessed on 10 January 2022).
- AOAC. Method 950.36, Protein in Bread. In Official Method of Analysis, 18th ed.; Association of the Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- VELP. Elemental Analysis and the Dumas Method. VELP Scientifica. 2020. Available online: https://www.velp.com/en-ww/dumas-method-1.aspx (accessed on 27 August 2021).
- Leitão, I.; Sales, J.; Martins, L.L.; Mourato, M.P. Response to stress induced by different potentially toxic elements (As, Cd, Cu and Na) in rapessed leaves. Plant Physiol. Rep. 2021, 26, 478–490. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Union: Bruxelles, Belgium, 2011; pp. 18–61. Available online: http://data.europa.eu/eli/reg/2011/1169/oj (accessed on 10 January 2022).
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant Properties and Phenolic Profile of the Most Widely Appreciated Cultivated Mushrooms: A Comparative Study between in Vivo and in Vitro Samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Mohankumar, J.B.; Uthira, L.; Su, M. Total Phenolic Content of Organic and Conventional Green Leafy Vegetables. J. Nutr. Hum. Health 2018, 2, 1088–1097. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Feldsine, P.T.; Leung, S.C.; Lienau, A.H.; Mui, L.A.; Townsend, D.E. Enumeration of total aerobic microorganisms in foods by SimPlate Total Plate Count-Color Indicator methods and conventional culture methods: Collaborative study. J. AOAC Int. 2003, 86, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Choi, S.K. Quality Characteristics of Muffins Containing Mealworm (Tenebrio molitor). Culin. Sci. Hosp. Res. 2015, 21, 104–115. [Google Scholar]
- Min, K.T.; Kang, M.S.; Kim, M.J.; Lee, S.H.; Han, J.S.; Kim, A.J. Manufacture and Quality Evaluation of Cookies prepared with Mealworm (Tenebrio molitor) Powder. Korean J. Food Nutr. 2016, 29, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, E.; Pankiewicz, U. Nutritional, Physiochemical, and Antioxidative Characteristics of Shortcake Biscuits Enriched with Tenebrio Molitor Flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef] [PubMed]
- Sai Manohar, R.; Haridas, R.P. Interrelationship between Rheological Characteristics of Dough and Quality of Biscuits; Use of Elastic Recovery of Dough to Predict Biscuit Quality. Food Res. Int. 2002, 35, 807–813. [Google Scholar] [CrossRef]
- Tunick, M.H.; Onwulata, C.I.; Thomas, A.E.; Phillips, J.G.; Mukhopadhyay, S.; Sheen, S.; Liu, C.K.; Latona, N.; Pimentel, M.R.; Cooke, P.H. Critical Evaluation of Crispy and Crunchy Textures: A Review. Int. J. Food Prop. 2013, 16, 949–963. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, E.; Pankiewicz, U.; Sujka, M. Nutritional, Physiochemical, and Biological Value of Muffins Enriched with Edible Insects Flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef]
- Arimi, J.M.; Duggan, E.; O’Sullivan, M.; Lyng, J.G.; O’Riordan, E.D. Effect of Water Activity on the Crispiness of a Biscuit (Crackerbread): Mechanical and Acoustic Evaluation. Food Res. Int. 2010, 43, 1650–1655. [Google Scholar] [CrossRef]
- Encina-Zelada, C.R.; Cadavez, V.; Monteiro, F.; Teixeira, J.A.; Gonzales-Barron, U. Combined Effect of Xanthan Gum and Water Content on Physicochemical and Textural Properties of Gluten-Free Batter and Bread. Food Res. Int. 2018, 111, 544–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravzanaadii, N.; Kim, S.H.; Choi, W.H.; Hong, S.H.; Kim, N.J. Nutritional Value of Mealworm, Tenebrio Molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef] [Green Version]
- European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, L12, 3–18. [Google Scholar]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio Molitor larvae as an alternative food source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, Antioxidant Activity, and Inhibitory Effect on Pancreatic Lipase of Extracts from the Edible Insects Acheta Domesticus and Tenebrio Molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of Functional Properties of Edible Insects and Protein Preparations Thereof. LWT Food Sci. Technol. 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Chatsuwan, N.; Nalinanon, S.; Puechkamut, Y.; Lamsal, B.P.; Pinsirodom, P. Characteristics, Functional Properties, and Antioxidant Activities of Water-Soluble Proteins Extracted from Grasshoppers, Patanga Succincta and Chondracris Roseapbrunner. J. Chem. 2018, 2018, 6528312. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Cheng, G.; Li, Y. Bread characteristics and antioxidant activities of Maillard reaction products of white pan bread containing various sugars. LWT Food Sci Technol. 2018, 95, 308–315. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial Counts of Mealworm Larvae (Tenebrio Molitor) and Crickets (Acheta Domesticus and Gryllodes Sigillatus) from Different Rearing Companies and Different Production Batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Crauwels, S.; Lievens, B.; Luca, S.; Claes, J.; Borremans, A.; Bruyninckx, L.; Van Campenhout, L. Effect of Post-Harvest Starvation and Rinsing on the Microbial Numbers and the Bacterial Community Composition of Mealworm Larvae (Tenebrio Molitor). Innov. Food Sci. Emerg. Technol. 2017, 42, 8–15. [Google Scholar] [CrossRef]
- Regulation (EC) No. 2073/2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2073&from=EN (accessed on 10 January 2022).
- SHC, FASFC. Food Safety Aspects of Insects Intended for Human Consumption (Sci Com Dossier 2014/04; SHC DOSSIER n° 9160). 2014. Available online: http://www.afsca.be/scientificcommittee/opinions/2014/_documents/Advice14-2014_ENG_DOSSIER2014-04.pdf (accessed on 10 January 2022).







| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| g/100 g | g/100 g | g/100 g | g/100 g | g/100 g | g/100 g | g/100g | |
| Wheat flour | 62 | 60 | 58 | 56 | 52 | 47 | 42 |
| Water | 28.5 | 28.5 | 28.5 | 28.5 | 28.5 | 28.5 | 28.5 |
| Oil | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 |
| Salt | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| T. molitor | 0.0 | 2.0 | 4.0 | 6.0 | 10.0 | 15.0 | 20.0 |
| Width (W) (mm) | Thickness (T) (mm) | Spread Ratio (W/T) | Density (g/cm3) | |
|---|---|---|---|---|
| Control cracker | 37.4 ± 0.52 a | 4.00 ± 0.76 a | 9.7 ± 1.8 a | 7.7 ± 1.7 a |
| Cracker T. molitor 2% | 37.5 ± 0.74 a | 3.467 ± 0.50 ab | 11,0 ± 1.5 ab | 8.2 ± 1.3 ab |
| Cracker T. molitor 4% | 38.0 ± 0.63 a | 3.34 ± 0.49 ac | 11.6 ± 1.9 ac | 8.8 ± 1.7 ab |
| Cracker T. molitor 6% | 37.3 ± 0.72 a | 3.03 ± 0.47 bcd | 12.6 ± 2.0 bcd | 9.1 ± 1.3 ab |
| Cracker T. molitor 10% | 37.8 ± 0.50 a | 2.75 ± 0.50 ce | 14.1 ± 2.3 cd | 9.1 ± 1.4 ab |
| Cracker T. molitor 15% | 37.1 ± 0.63 a | 2.47 ± 0.31 de | 15.2 ± 2.1 de | 9.2 ± 1.4 ab |
| Cracker T. molitor 20% | 37.2 ± 0.90 a | 2.17 ± 0.32 e | 17.5 ± 2.1 e | 9.7 ± 1.3 b |
| Water Content (g/100 g) | Water Activity (aw) | |
|---|---|---|
| Cracker control | 3.0 ± 0.1 a | 0.162 ± 0.008 a |
| Cracker T. molitor 6% | 1.8 ± 0.17 b | 0.132 ± 0.01 b |
| T. molitor flour | 6.3 ± 0.6 | 0.527 ± 0.001 |
| Ash (g/100 g) | Total Fat (g/100 g) | Protein (g/100 g) | Carbohydrate (g/100 g) * | Total Energy (Kcal/100 g) | |
|---|---|---|---|---|---|
| Cracker control | 1.90 ± 0.03 a | 12.7 ± 6.9 a | 9.65 ± 0.13 a | 72.7 | 443.9 |
| Cracker T. molitor 6% | 2.18 ± 0.03 b | 11.1 ± 0.5 a | 13.90 ± 0.65 b | 71.0 | 439.3 |
| T. molitor flour | 3.40 ± 0.01 | 20.0 ± 1.2 | 51.20 ± 1.76 | 19.3 | 462.0 |
| 15% RDV (mg/100 g) | Cracker Control (mg/100 g) | Cracker T. molitor 6% (mg/100 g) | T. molitor Flour (mg/100 g) | |
|---|---|---|---|---|
| Na | 300 | 639.93 ± 5.35 a | 656.80 ± 20.69 a | 131.47 ± 10.05 |
| K | 225 | 203.14 ± 2.10 a | 278.17 ± 6.24 b | 917.8 ± 10.71 |
| Ca | 120 | 20.89 ± 0.24 a | 22.60 ± 1.62 b | 55.63 ± 11.66 |
| Mg | 56.2 | 24.29 ± 0.02 a | 43.91 ± 2.07 b | 238.29 ± 4.41 |
| P | 105 | 96.23 ± 0.72 a | 152.14 ± 1.81 b | 740.09 ± 6.98 |
| Fe | 2.2 | 2.62 ± 1.69 a | 3.80 ± 1.66 a | 19.38 ± 21.75 |
| Cu | 0.2 | 0.24 ±0.04 a | 0.35 ± 0.005 b | 2.00 ± 0.07 |
| Zn | 1.5 | 0.83±0.02 a | 2.01 ± 0.04 b | 14.18 ± 0.23 |
| Mn | 0.4 | 0.78±0.01 a | 0.67 ± 0.02 b | 1.28 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djouadi, A.; Sales, J.R.; Carvalho, M.O.; Raymundo, A. Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour. Foods 2022, 11, 702. https://doi.org/10.3390/foods11050702
Djouadi A, Sales JR, Carvalho MO, Raymundo A. Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour. Foods. 2022; 11(5):702. https://doi.org/10.3390/foods11050702
Chicago/Turabian StyleDjouadi, Anna, Joana Rides Sales, Maria Otília Carvalho, and Anabela Raymundo. 2022. "Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour" Foods 11, no. 5: 702. https://doi.org/10.3390/foods11050702
APA StyleDjouadi, A., Sales, J. R., Carvalho, M. O., & Raymundo, A. (2022). Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour. Foods, 11(5), 702. https://doi.org/10.3390/foods11050702