Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality
Abstract
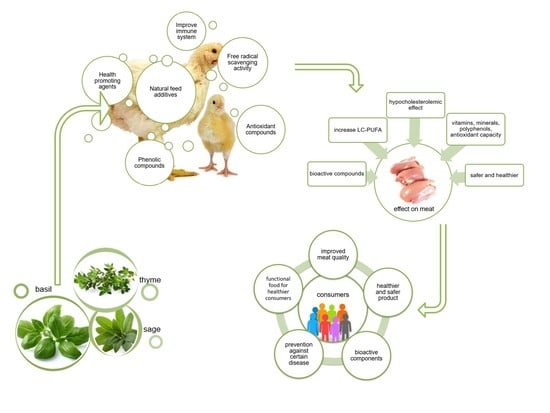
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Meat Sample Collection
2.4. Determination of Chemical Composition
2.5. Determination of Mineral Composition
2.6. Determination of Total Polyphenols Content and Antioxidant Capacity
2.7. Determination of Vitamin E, Lutein and Zeaxanthin
2.8. Determination of Fatty Acid and Cholesterol
2.9. Statistical Analysis
3. Results
3.1. Nutritional and Chemical Composition of the Plants
3.2. Effect of Dietary Plants on Chemical Composition of Chicken Thigh Meat
3.3. Effect of Dietary Plants on Antioxidant Activity in Chickens Thigh Meat
3.4. Effect of Dietary Plants on Fatty Acids Profile of Thigh Meat
3.5. Principal Component Analysis (PCA)
4. Discussion
4.1. Nutritional and Chemical Composition of Basil, Thyme and Sage Plants
4.2. Effect of Dietary Plants on Chemical and Mineral Composition of Chicken Thigh Meat
4.3. Effect of Plants on Antioxidant Compounds of Chicken Meat
4.4. Effect of Plants on Fatty Acid Composition and Cholesterol Content of Chicken Meat
4.5. Principal Component Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S.; Imran, A.; Hussain, M.B. Nutritional Composition of Meat. In Meat Science and Nutrition; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Admassu, S.; Kebede, M. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. 2019, 5, 38–49. [Google Scholar]
- Yu, H.H.; Chin, Y.-W.; Paik, H.-D. Application of natural preservatives for meat and meat products against food-borne pathogens and spoilage bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Substances Generally Recognized as Safe added to food for animals; Notice of Pilot Program. Fed. Regist. 2010, 75, 31800–31803. Available online: https://federalregister.gov/a/2010-13464 (accessed on 21 March 2022).
- Santoso, U.; Fenita, Y.; Kususiyah, K.; Widiantoro, O.; Kadarsih, S. The effect of medicinal herb on fat deposition, meat composition, amino acid and fatty acid composition of broiler meats. J. Indones. Trop. Anim. Agric. 2018, 43, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Pitino, R.; De Marchi, M.; Manuelian, C.L.; Johnson, M.; Simoni, M.; Righi, F.; Tsiplakou, E. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on yield, quality, and oxidative status of poultry products: A Review of the literature of the last 20 years. Antioxidants 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Grashorn, M.A. Use of phytobiotics in broiler nutrition–an alternative to infeed antibiotics. J. Anim. Feed Sci. 2010, 19, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Saracila, M.; Olteanu, M.; Panaite, T.D. Implications of using some phytoadditives in broiler nutrition—A review. Sci. Pap. Ser. D Anim. Sci. Int. Sess. Sci. Commun. Fac. Anim. Sci. 2020, 63, 165–172. [Google Scholar]
- Ahmadian, A.; Seidavi, A.; Phillips, C.J.C. Growth, Carcass Composition, Haematology and Immunity of Broilers Supplemented with Sumac Berries (Rhus coriaria L.) and Thyme (Thymus vulgaris). Animals 2020, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Panaite, T.D.; Untea, A.E.; Idriceanu, L.; Cornescu, G.M. Herbal plants as feed additives in broiler chicken diets. Arch. Zootech. 2021, 24, 76–95. [Google Scholar] [CrossRef]
- Akande, K.E.; Doma, U.D.; Agu, H.O.; Adamu, H.M. Major antinutrients found in plant protein sources: Their effect on nutrition. Pak. J. Nutr. 2010, 9, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Turcu, R.P. Effect of dietary orange and grapefruit peel on growth performance, health status, meat quality and intestinal microflora of broiler chickens. Ital. J. Anim. Sci. 2020, 19, 1394–1405. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Untea, A.; Criste, R.C.; Vladescu, L. Development and validation of a microwave digestion–FAAS procedure for Cu, Mn and Zn determination in liver. Rev. Chim. 2012, 63, 341–346. [Google Scholar]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, phosphomolybdenum assays for estimating antioxidant activity and phenolic compounds in five different plant extracts. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The Effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Varzaru, I.; Untea, A.E.; Van, I. Distribution of nutrients with benefic potential for the eyes in several medicinal plants. Rom. Biotechnol. Lett. 2015, 20, 10773–10783. [Google Scholar]
- Turcu, R.P.; Olteanu, M.; Criste, R.D.; Panaite, T.D.; Ropotă, M.; Vlaicu, P.A.; Drăgotoiu, D. Grapeseed meal used as natural antioxidant in high fatty acid diets for Hubbard broilers. Braz. J. Poult. Sci. 2019, 21, 001–012. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC Intl.: Rockville, MD, USA, 1996. [Google Scholar]
- Gurbuz, Y.; Ismael, I.A. Effect of peppermint and basil as feed additive on broiler performance and carcass characteristics. Iran. J. Appl. Anim. Sci. 2016, 6, 149–156. [Google Scholar]
- Gerencsér, Z.; Szendro, Z.; Matics, Z.; Radnai, I.; Kovács, M.; Nagy, I.; Dalle Zotte, A. Effect of dietary supplementation of spirulina (Arthrospira platensis) and thyme (Thymus vulgaris) on apparent digestibility and productive performance of growing rabbits. World Rabbit Sci. 2014, 22, 1–9. [Google Scholar] [CrossRef]
- Khalil, E.; Esoh, R.; Rababah, T.; Almajwal, A.M.; Alu, M.H. Minerals, proximate composition and their correlations of medicinal plants from Jordan. J. Med. Plants Res. 2012, 6, 5757–5762. [Google Scholar]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crops Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Lamari, Z.; Larbi, R.; Negache, H. Trace element content of Zingiber officinalis and Salvia officinalis medicinal plants from Algeria. J. Radioanal. Nucl. Chem. 2016, 309, 17–22. [Google Scholar] [CrossRef]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Benhima, R.; Amrani Joutei, K. Effects of salicylic acid on growth, mineral nutrition, glandular hairs distribution and essential oil composition in Salvia officinalis L. grown under copper stress. Environ. Sustain. 2020, 3, 199–208. [Google Scholar] [CrossRef]
- Koroch, A.R.; Juliani, H.R.; Sims, C.; Simon, J.E. Antioxidant activity, total phenolics, and rosmarinic acid content in different basils (Ocimum spp.). Isr. J. Plant Sci. 2010, 58, 191–195. [Google Scholar] [CrossRef]
- Kozlowska, M.; Laudy, A.E.; Przybyl, J.; Ziarno, M.; Majewska, E. Chemical composition and antibacterial activity of some medicinal plants from Lamiaceae family. Acta Pol. Pharm. 2015, 72, 757–767. [Google Scholar]
- Turcu, R.P.; Olteanu, M.; Untea, A.E.; Saracila, M.; Varzaru, I.; Vlaicu, P.A. Nutritional characterization of some natural plants used in poultry nutrition. Arch. Zootech. 2020, 23, 58–72. [Google Scholar] [CrossRef]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Shi, L.K.; Zhao, C.W.; Jin, Q.Z.; Wang, X.G. Fatty acid, phytochemical, oxidative stability and in vitro antioxidant property of sea buckthorn (Hippophaë rhamnoides L.) oils extracted by supercritical and subcritical technologies. LWT 2017, 86, 507–513. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Geçgel, Ü.; Gülcü, M.; Hamurcu, M.; Özcan, M.M. Bioactive properties, fatty acid composition and mineral contents of grape seed and oils. S. Afr. J. Enol. Vitic. 2017, 38, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ashour, E.A.; Abd El-Hack, M.E.; Swelum, A.A.; Osman, A.O.; Taha, A.E.; Alhimaidi, A.R.; Ismail, I.E. Does the dietary graded levels of herbal mixture powder impact growth, carcass traits, blood indices and meat quality of the broilers? Ital. J. Anim. Sci. 2020, 19, 1228–1237. [Google Scholar] [CrossRef]
- Cong, O.N.; Viet, D.N.; Kim, D.P.; Hornick, J.L. Effects of dietary sacha inchi (Plukenetia volubilis L.) oil and medicinal plant powder supplementation on growth performance, carcass traits, and breast meat quality of colored broiler chickens raised in Vietnam. Trop. Anim. Health Prod. 2022, 54, 87. [Google Scholar] [CrossRef] [PubMed]
- Herkrl, R.; Gálik, B.; Daniel, B.; Rolinec, M.; Šimko, M.; Juráček, M.; Wilkanowska, A. The effect of a phytogenic additive on nutritional composition of turkey meat. J. Cent. Eur. Agric. 2016, 17, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Stef, D.S.; Gergen, I. Effect of mineral-enriched diet and medicinal herbs on Fe, Mn, Zn, and Cu uptake in chicken. Chem. Cent. J. 2012, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Food and Agricultural Organization of the United Nations (FAO). Human Vitamin and Mineral Requirements. 2001. Available online: http://www.fao.org/3/a-y2809e.pdf (accessed on 23 March 2022).
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Untea, A.E.; Panaite, T.D.; Dragomir, C.; Ropota, M.; Olteanu, M.; Varzaru, I. Effect of dietary chromium supplementation on meat nutritional quality and antioxidant status from broilers fed with Camelina-meal-supplemented diets. Animal 2019, 13, 2939–2947. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Oude Griep, L.M.; Geleijnse, J.M.; Kromhout, D.; Ocké, M.C.; Verschuren, W.M.M. Raw and processed fruit and vegetable consumption and 10-year coronary heart disease incidence in a population-based cohort study in the netherlands. PLoS ONE 2010, 5, e13609. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; pp. 1–37. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a silver nanoparticle-based method for the antioxidant capacity measurement of polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef] [PubMed]
- Karre, L.; Lopez, K.; Getty, K.J. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.A.; Mancini-Filho, J.; Villavicencio, A.L.C.H. Natural antioxidants protecting irradiated beef burgers from lipid oxidation. LWT Food Sci. Technol. 2010, 43, 98–104. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- Mizi, L.; Cofrades, S.; Bou, R.; Pintado, T.; López-Caballero, M.E.; Zaidi, F.; Jiménez-Colmenero, F. Antimicrobial and antioxidant effects of combined high pressure processing and sage in beef burgers during prolonged chilled storage. Innov. Food Sci. Emerg. Technol. 2019, 51, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Lipiński, K.; Antoszkiewicz, Z.; Kotlarczyk, S.; Mazur-Kuśnirek, M.; Kaliniewicz, J.; Makowski, Z. The effect of herbal feed additive on the growth performance, carcass characteristics and meat quality of broiler chickens fed low-energy diets. Arch. Anim. Breed. 2019, 62, 33–40. [Google Scholar] [CrossRef]
- Luna, A.; Labaque, M.C.; Zygadlo, J.A.; Marin, R.H. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 2010, 89, 366–370. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Etherton, T.D. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 2000, 71, 179S–188S. [Google Scholar] [CrossRef] [Green Version]
- Mourão, J.L.; Pinheiro, V.M.; Prates, J.A.M.; Bessa, R.J.B.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Ponte, P.I.P. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poult. Sci. 2008, 87, 733–743. [Google Scholar] [CrossRef]
- Lanza, M.; Fasone, V.; Galofaro, V.; Barbagallo, D.; Bella, M.; Pennisi, P. Citrus pulp as an ingredient in ostrich diet: Effects on meat quality. Meat Sci. 2004, 68, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.E.; Kim, G.M.; Lee, S.K.; Yang, C.J. Growth performance, meat yield, oxidative stability, and Fatty Acid composition of meat from broilers fed diets supplemented with a medicinal plant and probiotics. Asian-Australas. J. Anim. Sci. 2012, 25, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Panaite, T.D. Effect of dietary pumpkin (Cucurbita moschata) seed meal on layer performance and egg quality characteristics. Anim. Biosci. 2022, 35, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Zoidis, E.; Simitzis, P.; Kampantais, D.; Katsoulas, P.; Pappas, A.C.; Papadomichelakis, G.; Goliomytis, M. Dietary Orange Pulp and Organic Selenium Effects on Growth Performance, Meat Quality, Fatty Acid Profile, and Oxidative Stability Parameters of Broiler Chickens. Sustainability 2022, 14, 1534. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F.J.L.P.S. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Dinh, T.T.; Thompson, L.D.; Galyean, M.L.; Brooks, J.C.; Patterson, K.Y.; Boylan, L.M. Cholesterol content and methods for cholesterol determination in meat and poultry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 269–289. [Google Scholar] [CrossRef]
- Al-Kassie, G.A. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak. Vet. J. 2009, 29, 169–173. [Google Scholar]
- Fallah, R.; Mirzaei, E. Effect of dietary inclusion of turmeric and thyme powders on performance, blood parameters and immune system of broiler chickens. Livest. Sci. 2016, 7, 180–186. [Google Scholar]
- Mariutti, L.R.B.; Nogueira, G.C.; Bragagnolo, N. Lipid and cholesterol oxidation in chicken meat are inhibited by sage but not by garlic. J. Food Sci. 2011, 76, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Stanaev, V.; Miloscaron, N.; Stanaev, V.; Plavscaron, N. Effect of garlic (Allium sativum L.) in fattening chicks nutrition. Afr. J. Agric. Res. 2011, 6, 943–948. [Google Scholar] [CrossRef]
- Hayajneh, F.M.F. Natural feed additives for broiler chickens. S. Afr. J. Anim. Sci. 2019, 49, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Raphaël, K.J.; Hervé, M.K.; Ruben, N.T.; Francklin, T.; Ronald, K.; Antoine, Y.; Alexis, T. Effect of dietary mimosa small bell (Dichostachys glomerata) fruit supplement as alternative to antibiotic growth promoter for broiler chicken. J. World’s Poul. Res. 2017, 7, 27–34. [Google Scholar]
- AbuMweis, S.S.; Marinangeli, C.P.; Frohlich, J.; Jones, P.J. Implementing phytosterols into medical practice as a cholesterol-lowering strategy: Overview of efficacy, effectiveness, and safety. Can. J. Cardiol. 2014, 30, 1225–1232. [Google Scholar] [CrossRef]



| Item | Basil | Thyme | Sage |
|---|---|---|---|
| Chemical Composition * | |||
| Dry matter, % | 91.35 | 91.65 | 90.64 |
| Crude protein, % | 22.53 | 15.38 | 9.56 |
| Crude fat, % | 1.51 | 2.09 | 3.15 |
| Crude fibre, % | 12.22 | 17.08 | 27.92 |
| Ash, % | 14.12 | 9.43 | 10.36 |
| Mineral Composition * | |||
| Copper, mg/kg | 27.69 | 7.41 | 7.89 |
| Iron, mg/kg | 624.51 | 690.05 | 732.72 |
| Manganese, mg/kg | 78.46 | 96.11 | 68.92 |
| Zinc, mg/kg | 54.63 | 31.73 | 38.87 |
| Fatty Acids, g/100 g | Basil | Thyme | Sage |
|---|---|---|---|
| Caproic C6:0 | 0.48 | 0.36 | 1.72 |
| Caprylic C8:0 | 0.45 | 0.38 | 6.88 |
| Capric C10:0 | 0.75 | 0.51 | 0.39 |
| Lauric C12:0 | 1.92 | 0.94 | 2.38 |
| Myristic C14:0 | 5.20 | 21.41 | 0.43 |
| Pentadecanoic C15:0 | 0.59 | nd | 0.38 |
| Palmitic C16:0 | 22.98 | 17.12 | 21.38 |
| Heptadecanoic C17:0 | nd | 0.08 | 0.49 |
| Stearic C18:0 | 8.16 | 3.06 | 4.10 |
| Tricosanoic C23:0 | 0.00 | 0.00 | 0.64 |
| SFA | 40.52 | 43.86 | 38.79 |
| Miristioleic C14:1 | 0.73 | 1.59 | 0.27 |
| Pentadecenoic C15:1 | 1.41 | 1.23 | 3.23 |
| Palmitoleic C16:1 | 1.99 | 0.89 | 2.46 |
| Heptadecenoic C17:1 | nd | 0.06 | 0.29 |
| Oleic cis C18:1 | 17.85 | 7.54 | 12.65 |
| Nervonic C24:1n9 | 0.00 | 0.68 | 0.80 |
| MUFA | 21.99 | 11.98 | 19.70 |
| Linoleic cis C18:2n6 | 17.36 | 12.62 | 11.40 |
| Linolenic γ C18:3n6 | nd | 0.16 | nd |
| Eicosadienoic C20:2n6 | nd | 0.17 | 3.21 |
| Eicosatrienoic C20:3n6 | nd | nd | 3.48 |
| Arachidonic C20:4n6 | 0.55 | 0.46 | 5.00 |
| n-6 PUFA | 17.91 | 13.41 | 23.08 |
| α-Linolenic C18:3n3 | 15.95 | 27.96 | 12.61 |
| Octadecatetraenoic C18:4n3 | 2.71 | 0.90 | 5.27 |
| Eicosapentaenoic C20:5n3 | nd | 0.92 | nd |
| n-3 PUFA | 18.66 | 29.78 | 17.87 |
| PUFA | 36.57 | 43.19 | 40.96 |
| Others | 0.92 | 0.97 | 0.56 |
| n-6/n-3 ratio | 0.96 | 0.45 | 1.29 |
| Item | C | B | T | S | SEM | p |
|---|---|---|---|---|---|---|
| Chemical Composition | ||||||
| Dry matter, % | 28.23 | 29.53 | 28.08 | 28.78 | 0.549 | 0.6568 |
| Crude protein, % | 18.33 | 18.18 | 18.06 | 18.23 | 0.321 | 0.9938 |
| Crude fat, % | 8.36 | 7.87 | 8.08 | 7.88 | 0.291 | 0.0671 |
| Ash, % | 1.11 | 1.15 | 1.14 | 1.06 | 0.023 | 0.8591 |
| Mineral Composition | ||||||
| Copper, mg/kg | 1.10 | 1.27 | 1.07 | 1.14 | 0.058 | 0.8592 |
| Iron, mg/kg | 38.14 | 41.00 | 41.28 | 41.19 | 0.723 | 0.2592 |
| Manganese, mg/kg | 0.08 | 0.11 | 0.09 | 0.10 | 0.022 | 0.1056 |
| Zinc, mg/kg | 50.63 b | 58.52 a | 54.92 a | 54.79 a | 1.075 | 0.0408 |
| Fatty Acids, g/100 g | C | B | T | S | SEM | p |
|---|---|---|---|---|---|---|
| Butyric C4:0 | 0.170 a | 0.123 b | 0.121 b | 0.090 c | 0.007 | <0.0001 |
| Caproic C6:0 | 0.122 a | 0.102 b | 0.107 b | 0.083 b | 0.004 | 0.0018 |
| Caprylic C8:0 | 0.352 a | 0.138 b | 0.063 c | 0.073 c | 0.021 | <0.0001 |
| Capric C10:0 | 0.288 a | 0.113 b | 0.093 bc | 0.048 c | 0.019 | <0.0001 |
| Lauric C12:0 | 0.04 | 0.03 | 0.03 | 0.03 | 0.003 | 0.6454 |
| Myristic C14:0 | 1.040 a | 0.720 b | 0.623 b | 0.528 c | 0.042 | <0.0001 |
| Pentadecanoic C15:0 | 0.455 a | 0.327 b | 0.378 b | 0.367 b | 0.013 | 0.0336 |
| Palmitic C16:0 | 27.07 a | 26.37 a | 26.12 a | 23.13 b | 0.333 | 0.0002 |
| Heptadecanoic C17:0 | 0.08 | 0.27 | 0.18 | 0.17 | 0.023 | 0.0948 |
| Stearic C18:0 | 7.652 b | 8.705 a | 7.878 b | 8.095 b | 0.109 | 0.0009 |
| Lignoceric C24:0 | 0.563 b | 0.530 b | 0.637 ab | 0.697 a | 0.020 | 0.0280 |
| SFA | 37.84 a | 37.428 a | 36.227 a | 33.312 b | 0.411 | 0.0009 |
| Miristoleic C14:1 | 0.278 a | 0.232 ab | 0.242 a | 0.153 b | 0.083 | 0.0287 |
| Pentadecenoic C15:1 | 1.59 | 1.84 | 1.48 | 1.18 | 0.075 | 0.0678 |
| Palmitoleic C16:1 | 5.203 a | 4.157 b | 4.983 ab | 3.643 c | 0.117 | <0.0001 |
| Heptadecenoic C17:1 | 0.23 | 0.28 | 0.28 | 0.24 | 0.018 | 0.5986 |
| Oleic cis C18:1n9 | 40.24 a | 39.62 a | 39.75 a | 35.86 b | 0.460 | 0.0040 |
| Erucic C22:1n9 | 2.695 a | 3.222 a | 2.663 a | 1.708 b | 0.142 | 0.0032 |
| Nervonic C24:1n9 | 0.092 b | 0.057 b | 0.092 b | 0.235 a | 0.018 | 0.0046 |
| MUFA | 50.32 a | 49.40 a | 49.49 a | 43.03 b | 0.713 | 0.0007 |
| Linoleic cis (LA) C18:2n6 | 5.695 b | 5.950 b | 7.193 a | 7.150 a | 0.201 | 0.0482 |
| Linolenic γ C18:3n6 | 0.09 | 0.05 | 0.11 | 0.07 | 0.012 | 0.7736 |
| Conjugated LA C18:2 | 0.50 | 0.52 | 0.42 | 0.31 | 0.026 | 0.0782 |
| Eicosadienoic C20:2n6 | 0.28 | 0.32 | 0.24 | 0.24 | 0.013 | 0.2434 |
| Eicosatrienoic C20:3n6 | 0.12 | 0.17 | 0.11 | 0.14 | 0.008 | 0.1917 |
| Arachidonic C20:4n6 | 0.092 b | 0.110 b | 0.103 b | 0.820 a | 0.083 | 0.0088 |
| Docosadienoic C22:2n6 | 0.327 b | 0.373 b | 0.485 a | 0.465 a | 0.015 | 0.0001 |
| Docosatrienoic C22:3n6 | 0.37 | 0.43 | 0.51 | 0.45 | 0.017 | 0.1213 |
| Docosatetraenoic C22:4n6 | 0.22 | 0.16 | 0.31 | 0.20 | 0.032 | 0.4246 |
| n-6 PUFA | 7.617 b | 8.062 b | 9.445 a | 9.623 a | 0.223 | 0.0025 |
| α-Linolenic (ALA) C18:3n3 | 0.090 c | 0.240 b | 0.263 b | 0.333 a | 0.021 | 0.0350 |
| Octadecatetraenoic C18:4n3 | 0.81 | 0.89 | 0.67 | 0.59 | 0.043 | 0.1228 |
| Eicosatrienoic C20:3n3 | 0.058 b | 0.080 b | 0.221 a | 0.230 a | 0.017 | 0.0032 |
| Eicosapentaenoic (EPA) C20:5n3 | 0.443 b | 0.523 a | 0.533 a | 0.557 a | 0.015 | 0.0081 |
| Docosapentaenoic C22:5n3 | 0.093 b | 0.223 a | 0.340 a | 0.390 a | 0.045 | 0.0452 |
| Docosahexaenoic (DHA) C22:6n3 | 0.130 b | 0.332 a | 0.372 a | 0.350 a | 0.031 | 0.0160 |
| n-3 PUFA | 1.614 b | 2.283 a | 2.394 a | 2.450 a | 0.068 | <0.0001 |
| PUFA | 9.240 b | 10.35 a | 11.72 a | 12.07 a | 0.261 | 0.0023 |
| others | 2.621 b | 2.823 a | 2.718 a | 2.020 b | 0.141 | 0.0189 |
| n-6/n-3 ratio | 4.719 a | 3.531 b | 3.945 b | 3.927 b | 0.164 | 0.0310 |
| PUFA/SFA | 0.24 | 0.28 | 0.32 | 0.36 | 0.144 | 0.0615 |
| Cholesterol, mg/100g DM | 60.50 a | 45.90 b | 41.60 b | 48.40 b | 0.002 | 0.0007 |
| H/H | 1.66 b | 1.73 a | 1.79 a | 1.88 a | 0.033 | 0.0302 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality. Foods 2022, 11, 1105. https://doi.org/10.3390/foods11081105
Vlaicu PA, Untea AE, Turcu RP, Saracila M, Panaite TD, Cornescu GM. Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality. Foods. 2022; 11(8):1105. https://doi.org/10.3390/foods11081105
Chicago/Turabian StyleVlaicu, Petru Alexandru, Arabela Elena Untea, Raluca Paula Turcu, Mihaela Saracila, Tatiana Dumitra Panaite, and Gabriela Maria Cornescu. 2022. "Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality" Foods 11, no. 8: 1105. https://doi.org/10.3390/foods11081105
APA StyleVlaicu, P. A., Untea, A. E., Turcu, R. P., Saracila, M., Panaite, T. D., & Cornescu, G. M. (2022). Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality. Foods, 11(8), 1105. https://doi.org/10.3390/foods11081105